Hemoglobinopathies
Hemoglobinopathies
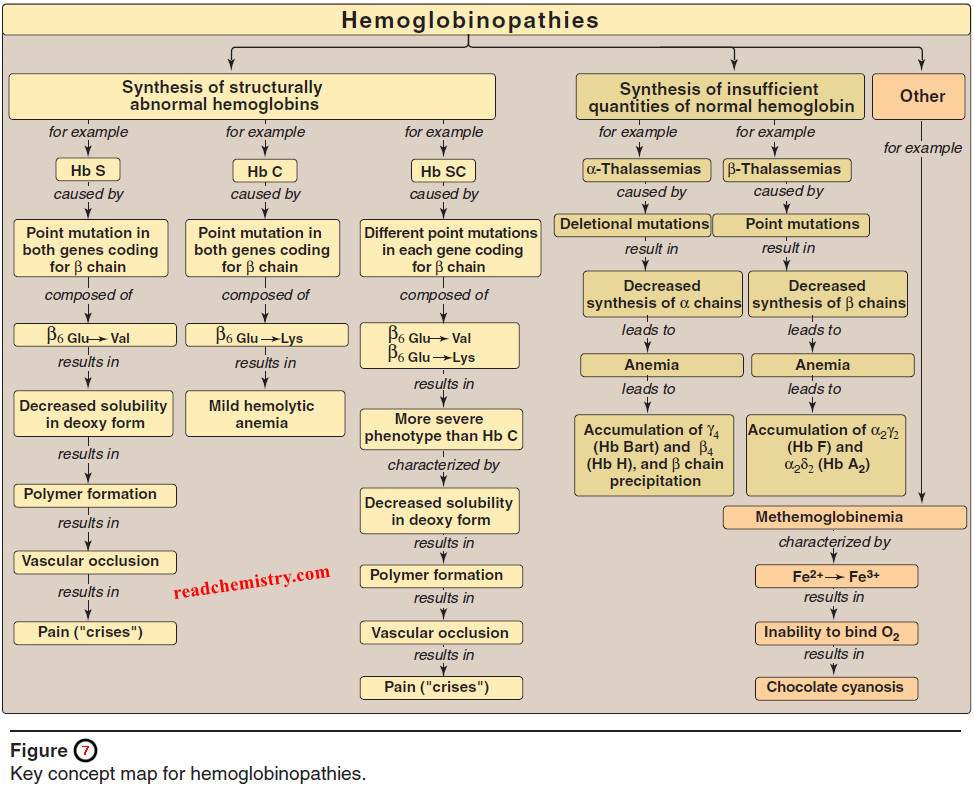
– Hemoglobinopathies have traditionally been defined as a family of genetic disorders caused by production of a structurally abnormal hemoglobin molecule, synthesis of insufficient quantities of normal hemoglobin, or, rarely, both.
– Sickle cell anemia (Hb S), hemoglobin C disease (Hb C), hemoglobin SC disease (Hb S + Hb C), and the thalassemia syndromes are representative hemoglobinopathies that can have severe clinical consequences.
– The first three conditions result from production of hemoglobin with an altered amino acid sequence (qualitative hemoglobinopathy), whereas the thal assemias are caused by decreased production of normal hemoglobin (quantitative hemo – globinopathy).
A. Sickle cell anemia (hemoglobin S disease)
– Sickle cell anemia, the most common of the red cell sickling diseases, is a genetic disorder of the blood caused by a single nucleotide alteration (a point mutation) in the gene for β-globin. It is the most common inherited blood disorder in the United States, affecting 80,000 Americans.
– It occurs primarily in the African –American population, affecting one of 500 newborn African-American infants in the United States.
– Sickle cell anemia is a homozygous, recessive disorder.
– It occurs in individuals who have inherited two mutant genes (one from each parent) that code for synthesis of the β chains of the globin molecules.
– [Note: The mutant β-globin chain is designated βS, and the resulting hemoglobin, α2βS2, is referred to as Hb S.]
– An infant does not begin showing symptoms of the disease until sufficient Hb F has been replaced by Hb S so that sickling can occur (see below).
– Sickle cell anemia is characterized by lifelong episodes of pain (“crises”), chronic hemolytic anemia with associated hyperbilirubinemia, and increased susceptibility to infections, usually beginning in early childhood.
– [Note: The lifetime of an erythrocyte in sickle cell anemia is less than 20 days, compared with 120 days for normal RBCs; hence, the anemia.]
– Other symptoms include acute chest syndrome, stroke, splenic and renal dysfunction, and bone changes due to marrow hyperplasia.
– Heterozygotes, representing 1 in 12 African-Americans, have one normal and one sickle cell gene.
– The blood cells of such heterozygotes contain both Hb S and Hb A. These individuals have sickle cell trait.
– They usually do not show clinical symptoms and can have a normal life span.
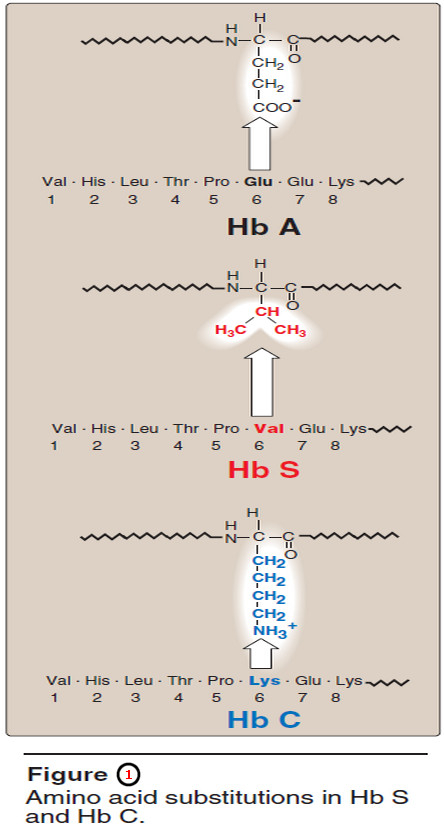
1. Amino acid substitution in Hb S β chains
– A molecule of Hb S contains two normal α-globin chains and two mutant β-globin chains (βS), in which glutamate at position six has been replaced with valine (Figure 1).
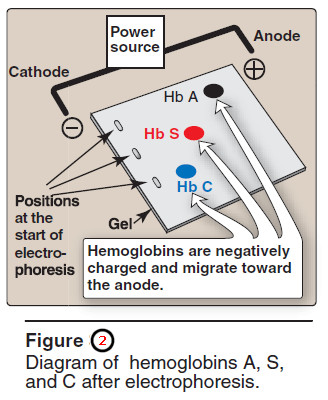
– Therefore, during electrophoresis at alkaline pH, Hb S migrates more slowly toward the anode (positive electrode) than does Hb A (Figure 2).
– This altered mobility of Hb S is a result of the absence of the negatively charged glutamate residues in the two β chains, thus rendering Hb S less negative than Hb A.
– [Note: Electrophoresis of hemoglobin obtained from lysed RBCs is routinely used in the diagnosis of sickle cell trait and sickle cell disease.]
2. Sickling and tissue anoxia
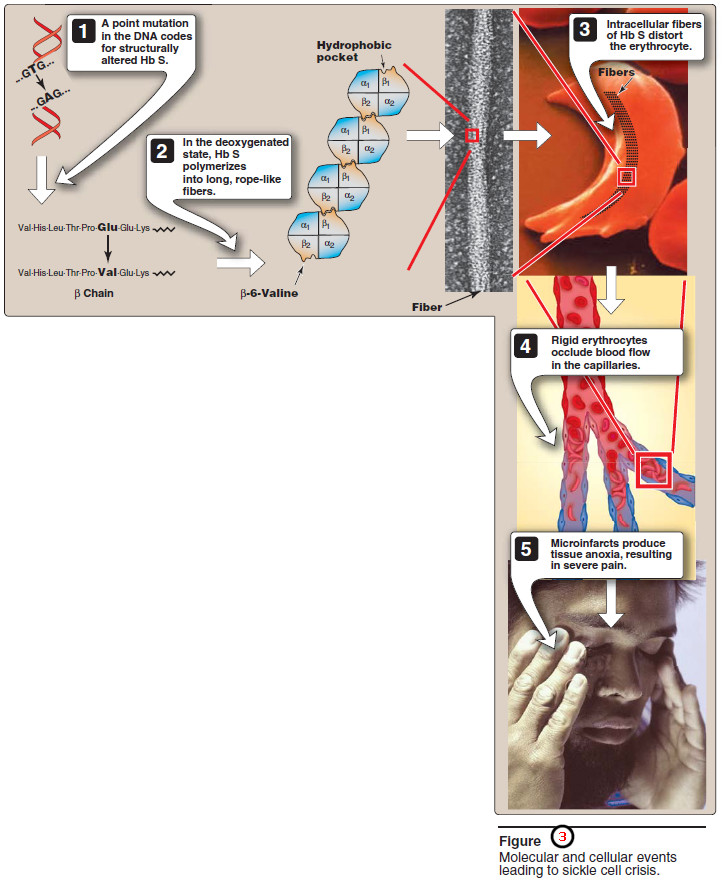
– The replacement of the charged glutamate with the nonpolar valine forms a protrusion on the β-globin that fits into a complementary site on the β chain of another hemoglobin molecule in the cell (Figure 3).
– At low oxygen tension, deoxyhemoglobin S polymerizes inside the RBC, forming a network of fibrous polymers that stiffen and distort the cell, producing rigid, misshapen erythrocytes.
– Such sickled cells frequently block the flow of blood in the narrow capillaries.
– This interruption in the supply of oxygen leads to localized anoxia (oxygen deprivation) in the tissue, causing pain and eventually death (infarction) of cells in the vicinity of the blockage.
– The anoxia also leads to an increase in deoxygenated Hb S.
– [Note: The mean diameter of RBCs is 7.5 μm, whereas that of the microvasculature is 3–4 μm.
– Instead of squeezing through the microvasculature like Hb A containing RBCs, sickled cells have a decreased ability to deform and an increased tendency to adhere to vessel walls, and so have difficulty moving through small vessels.]
3. Variables that increase sickling
– The extent of sickling and, therefore, the severity of disease is enhanced by any variable that increases the proportion of Hb S in the deoxy state (that is, reduces the affinity of Hb S for oxygen).
– These variables include decreased oxygen tension as a result of high altitudes or flying in a nonpressurized plane, increased pCO2, decreased pH, dehydration, and an increased concentration of 2,3- BPG in erythrocytes.
4. Treatment
– Therapy involves adequate hydration, analgesics, aggressive antibiotictherapy if infection is present, and transfusions in patients at high risk for fatal occlusion of blood vessels.
– Intermittent transfusions with packed red cells reduce the risk of stroke, but the benefits must be weighed against the complications of transfusion, which include iron overload (hemosiderosis), bloodborne infections, and immunologic complications.
– Hydroxyurea, an antitumor drug, is therapeutically useful because it increases circulating levels of Hb F, which decreases RBC sickling.
– This leads to decreased frequency of painful crises and reduces mortality.
5. Possible selective advantage of the heterozygous state
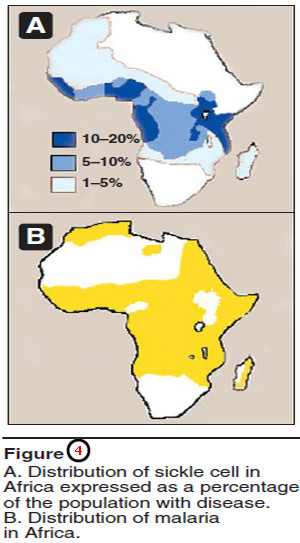
– The high frequency of the βS mutation among black Africans, despite its damaging effects in the homozygous state, suggests that a selective advantage exists for heterozygous individuals.
– For example, heterozygotes for the sickle cell gene are less susceptible to malaria, caused by the parasite Plasmodium falciparum.
– This organism spends an obligatory part of its life cycle in the RBC.
– One theory is that because these cells in individuals heterozygous for Hb S, like those in homozygotes, have a shorter life span than normal, the parasite cannot complete the intracellular stage of its development.
– This fact may provide a selective advantage to hetero zygotes living in regions where malaria is a major cause of death.
– Figure (4) illustrates that in Africa, the geographic distribution of sickle cell anemia is similar to that of malaria.
– The morbidity and mortality associated with sickle cell anemia has led to its inclusion in newborn screening panels to allow prophylactic antibiotic therapy to begin soon after the birth of an affected child.
B. Hemoglobin C disease
– Like Hb S, Hb C is a hemoglobin variant that has a single amino acid substitution in the sixth position of the β-globin chain (see Figure 1).
– In this case, however, a lysine is substituted for the glutamate (as compared with a valine substitution in Hb S).
– [Note: This substitution causes Hb C to move more slowly toward the anode than Hb A or Hb S does (see Figure 2).]
– Patients homo – zygous for hemoglobin C generally have a relatively mild, chronic hemolytic anemia.
– These patients do not suffer from infarctive crises, and no specific therapy is required.
C. Hemoglobin SC disease
– Hemoglobin SC disease is another of the red cell sickling diseases.
– In this disease, some β-globin chains have the sickle cell mutation, whereas other β-globin chains carry the mutation found in Hb C disease.
– [Note: Patients with Hb SC disease are doubly heterozygous (compound heterozygote) because both of their β-globin genes are abnormal, although different from each other.]
– Hemoglobin levels tend to be higher in Hb SC disease than in sickle cell anemia, and may even be at the low end of the normal range.
– The clinical course of adults with Hb SC anemia differs from that of sickle cell anemia in that symptoms such as painful crises are less frequent and less severe; however, there is significant clinical variability.
D. Methemoglobinemias
Oxidation of the heme component of hemoglobin to the ferric (Fe3+) state forms methemoglobin, which cannot bind oxygen.
– This oxidation may be caused by the action of certain drugs, such as nitrates, or endogenous products, such as reactive oxygen intermediates.
– The oxidation may also result from inherited defects, for example, certain mutations in the α- or β-globin chain promote the formation of methemoglobin (Hb M).
– Furthermore, a deficiency of NADH-cytochrome b5 reductase (also called NADH-met hemoglobin reductase), the enzyme responsible for the conversion of methemoglobin (Fe3+) to hemoglobin (Fe2+), leads to the accumulation of met hemoglobin.
– [Note: The erythrocytes of newborns have approximately half the capacity of those of adults to reduce methemoglobin.
– They are therefore particularly susceptible to the effects of met – hemoglobin-producing compounds.]
– The methemoglobin emias are characterized by “chocolate cyanosis” (a brownish-blue coloration of the skin and mucous membranes) and chocolate-colored blood, as a result of the dark-colored methemoglobin.
– Symptoms are related to the degree of tissue hypoxia, and include anxiety, headache, and dyspnea.
– In rare cases, coma and death can occur.
– Treatment is with methylene blue, which is oxidized as Fe+3 is reduced.
E. Thalassemias
– The thalassemias are hereditary hemolytic diseases in which an imbalance occurs in the synthesis of globin chains.
– As a group, they are the most common single gene disorders in humans.
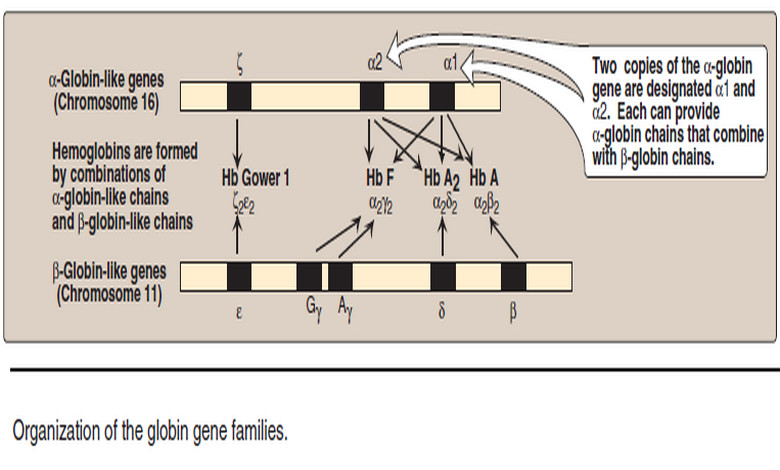
– Normally, synthesis of the α- and β-globin chains is coordinated, so that each α-globin chain has a β-globin chain partner. This leads to the formation of α2β2 (Hb A).
– In the tha l assemias, the synthesis of either the α- or the β-globin chain is defective.
– A thalassemia can be caused by a variety of mutations, including entire gene deletions, or substitutions or deletions of one to many nucleo tides in the DNA.
– [Note: Each thalassemia can be classified as either a disorder in which no globin chains are produced (αo – or βo – thal assemia), or one in which some chains are synthesized, but at a reduced level (α+– or β+ thalassemia).]
1. β-Thalassemias
– In these disorders, synthesis of β-globin chains is decreased or absent, typically as a result of point mutations that affect the production of functional mRNA; however, α-globin chain synthesis is normal.
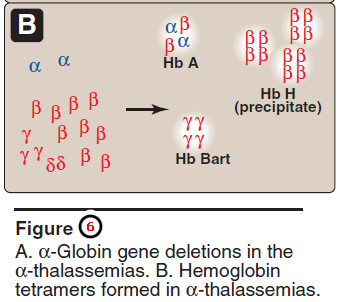
– α Globin chains cannot form stable tetramers and, therefore, precipitate, causing the premature death of cells initially destined to become mature RBCs.
– Increase in α2γ2 (Hb F) and α2δ2 (Hb A2) also occurs.
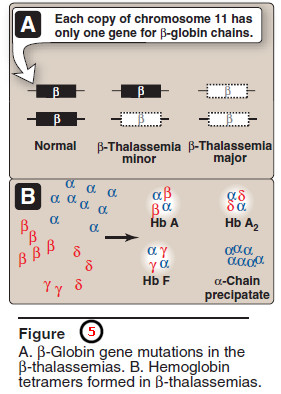
– There are only two copies of the β-globin gene in each cell (one on each chromosome 11).
– Therefore, individuals with β-globin gene defects have either β-thal assemia trait (β-thalassemia minor) if they have only one defective β globin gene, or β-thalassemia major (Cooley anemia if both genes are defective (Figure 5).
– Because the β-globin gene is not expressed until late in fetal gestation, the physical manifestations of β-thalassemias appear only several months after birth.
– Those individuals with β-thalassemia minor make some β chains, and usually do not require specific treatment.
– However, those infants born with β-thal assemia major are seemingly healthy at birth, but become severely anemic, usually during the first or second year of life due to ineffective erythropoiesis.
– Skeletal changes as a result of extramedullary hematopoiesis also are seen.These patients require regular trans fusions of blood.
– [Note: Although this treatment is lifesaving, the cumulative effect of the transfusions is iron overload (a syndrome known as hemosiderosis).
– Use of iron chelation therapy has improved morbidity and mortality.]
– The increasing use of bone marrow replacement therapy has been a boon to these patients.
2. α-Thalassemias
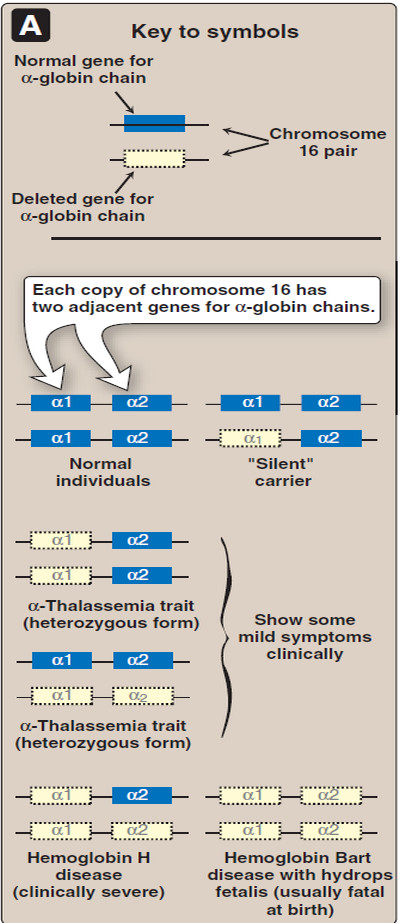
– These are defects in which the synthesis of α-globin chains is decreased or absent, typically as a result of deletional mutations.
– Because each individual’s genome contains four copies of the α-globin gene (two on each chromosome 16), there are several levels of α-globin chain deficiencies.
– If one of the four genes is defective, the individual is termed a silent carrier of α-thalassemia, because no physical manifestations of the disease occur.
– If two α-globin genes are defective, the individual is designated as having α -thal assemia trait.
– If three α-globin genes are defective, the individual has Hb H (β4) disease—a mildly to moderately severe hemolytic anemia.
– If all four α-globin genes are defective, Hb Bart (γ4) disease with hydrops fetalis and fetal death results, because α-globin chains are required for the synthesis of Hb F.
– [Note: Hemoglobinopathies that result in increased O2 affinity typically are characterized by increased production of RBCs, whereas those that result in decreased O2 affinity are characterized by anemia.]
References
- Lehninger Principles of Biochemistry / David L. Nelson, Michael M. Cox/ 7th ed, 2017.
- Lippincott’s Illustrated Reviews: Biochemistry / Richard A. Harvey, Denise R. Ferrier/ 5th ed, 2011 / Lippincott Williams & Wilkins, USA.
- Harper’s Illustrated Biochemistry /Robert K. Murray, David A. Bender , Kathleen M. Botham / 28th, 2009/ McGraw-Hill Companies, Inc. USA.