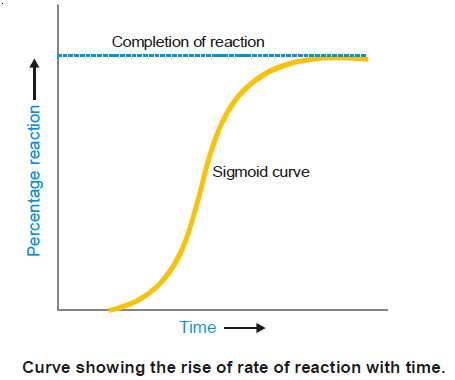
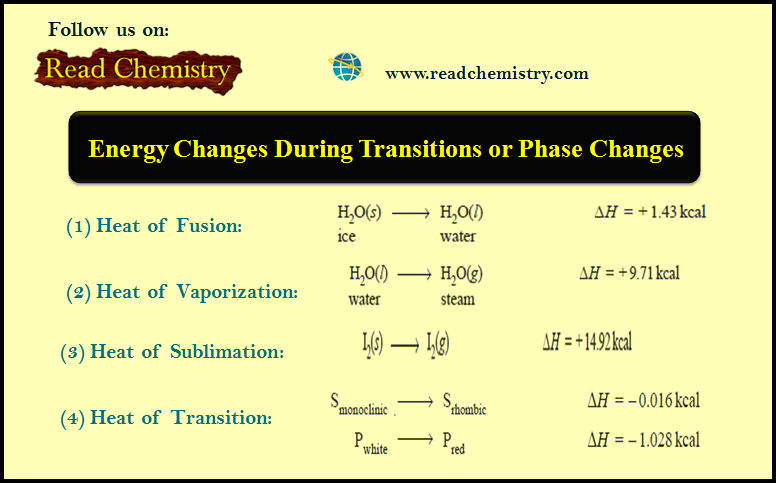
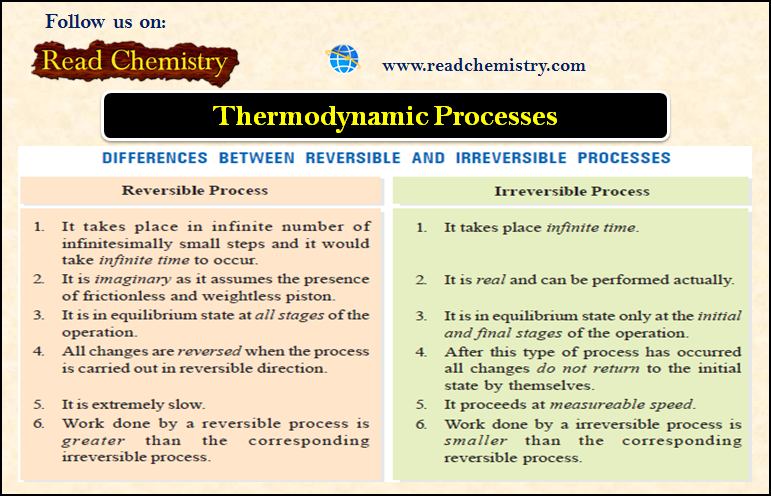
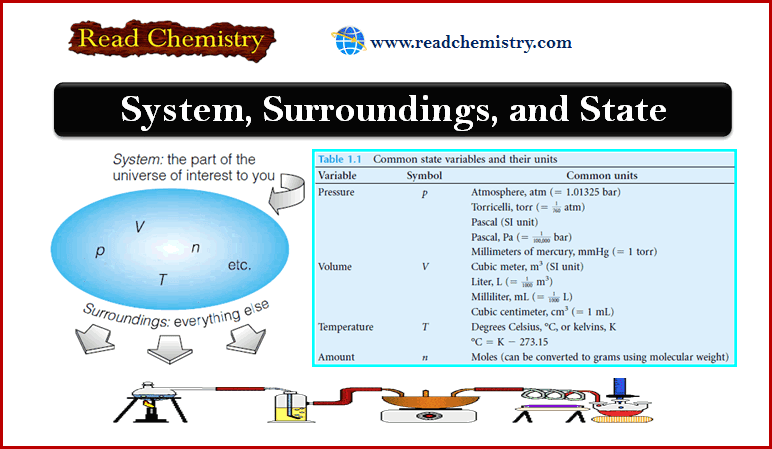
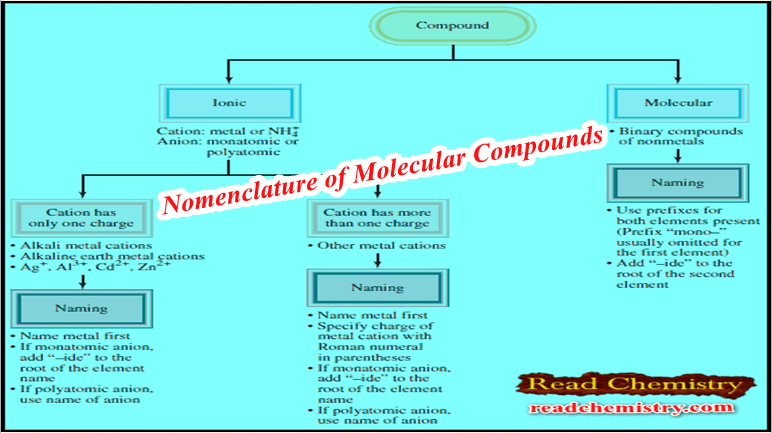
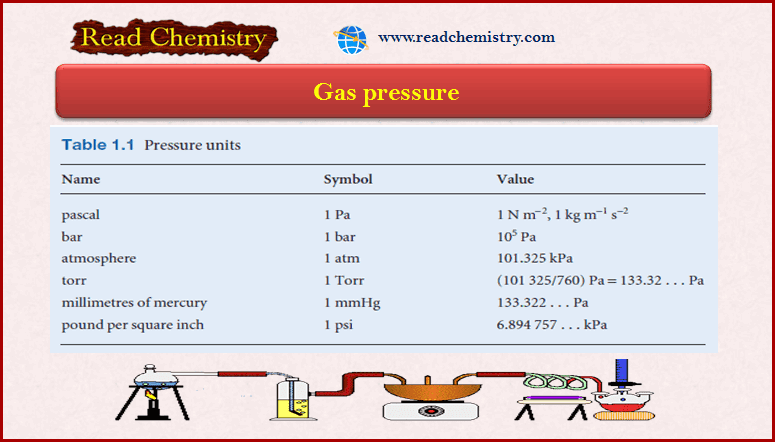
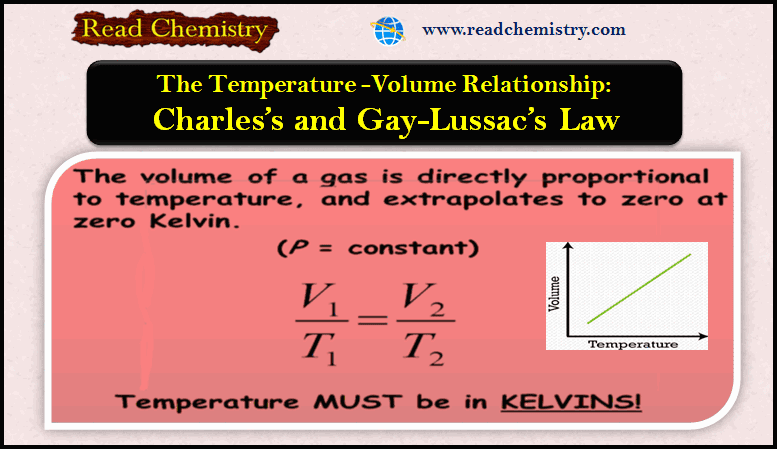
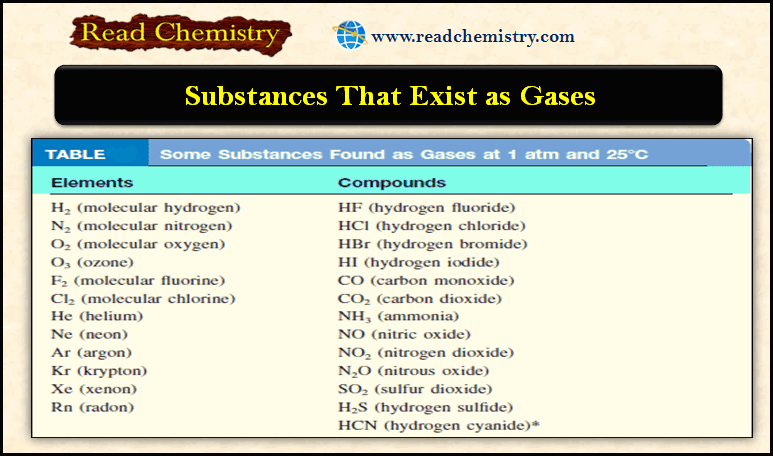
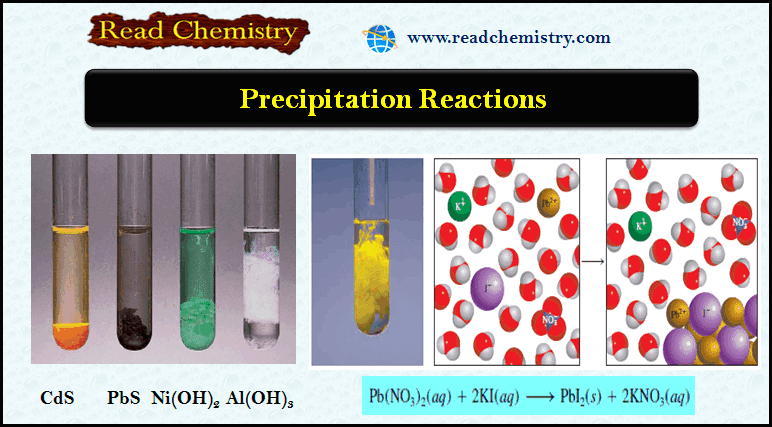
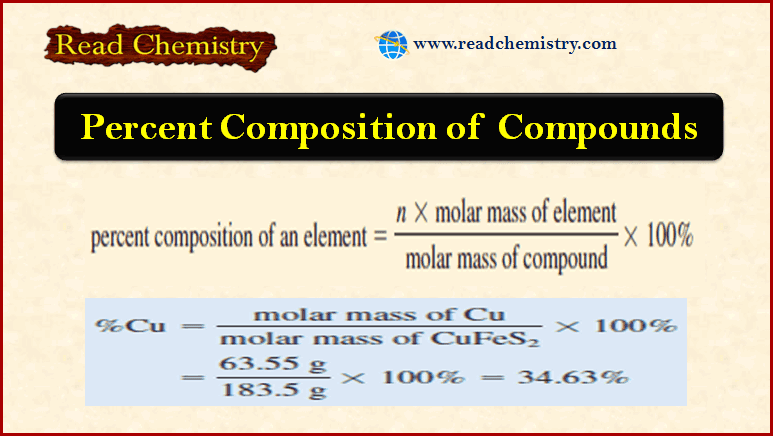
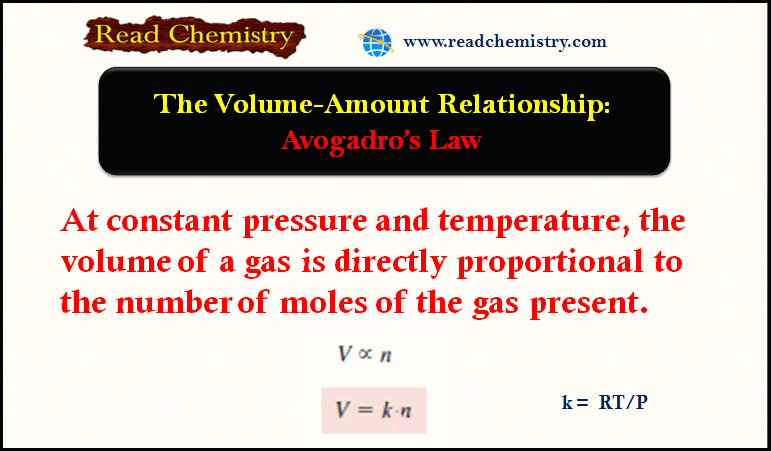
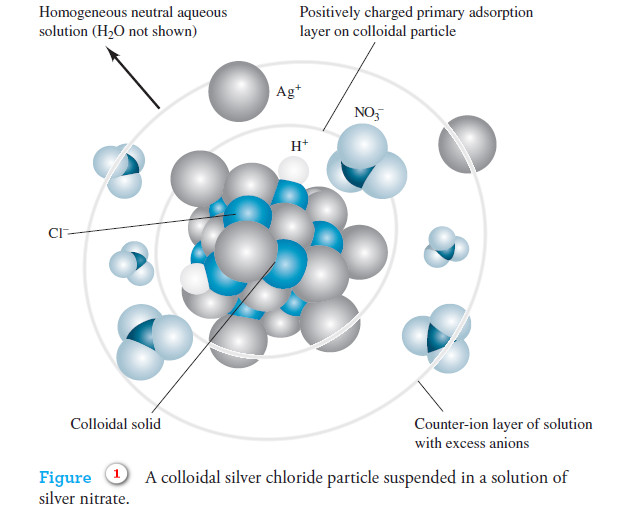
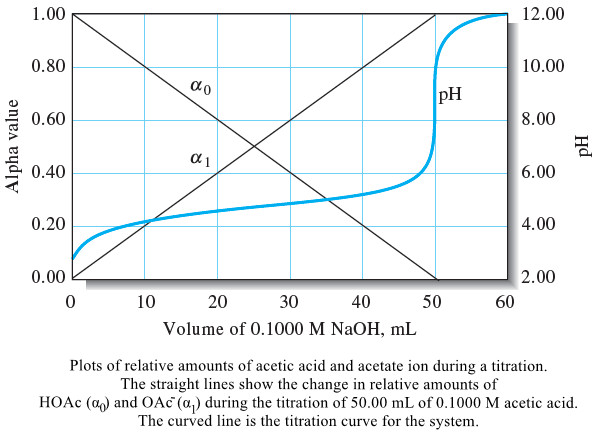
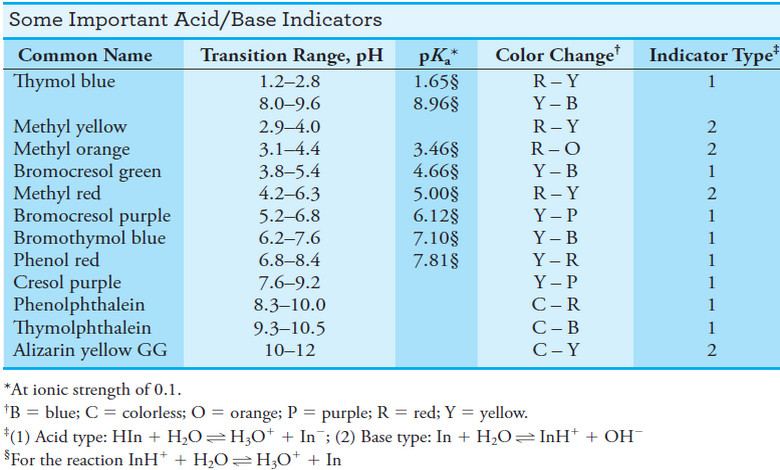
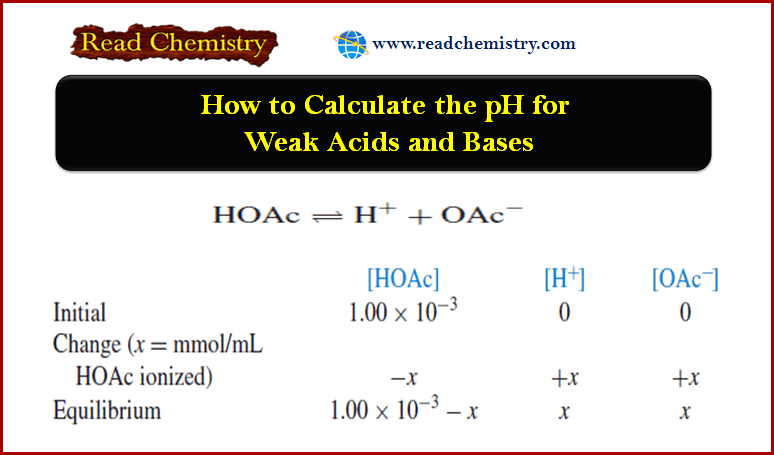
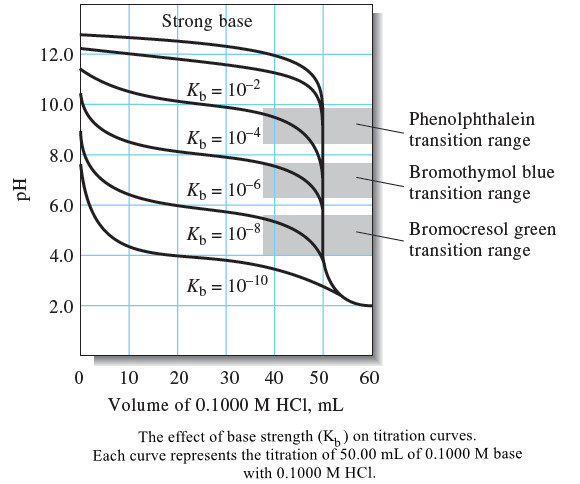
Popular Posts
-
General Chemistry
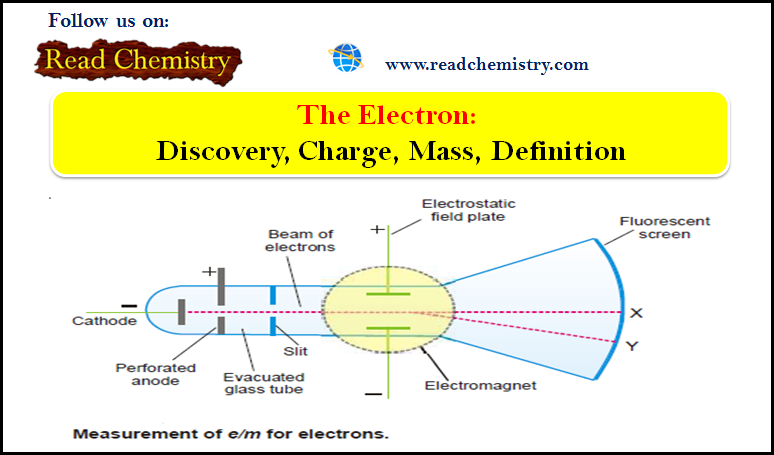
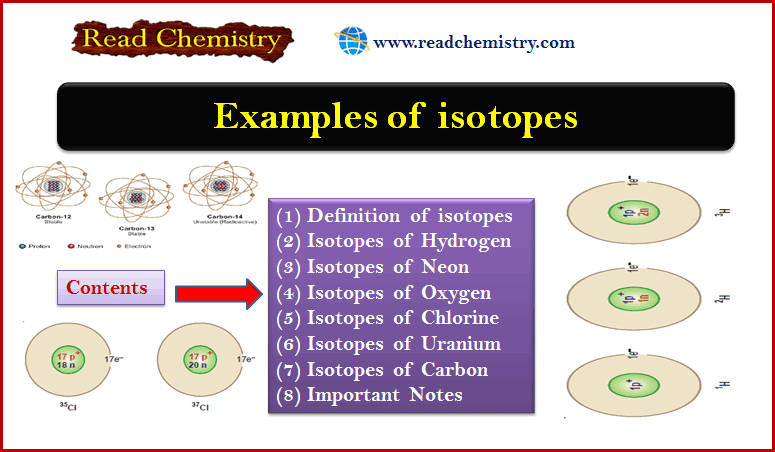
Electron: Discovery, Charge, Mass, Definition
Cathode Rays – The discovery of electron – The knowledge about the electron was derived as a result of the…
Read More » -
Physical Chemistry
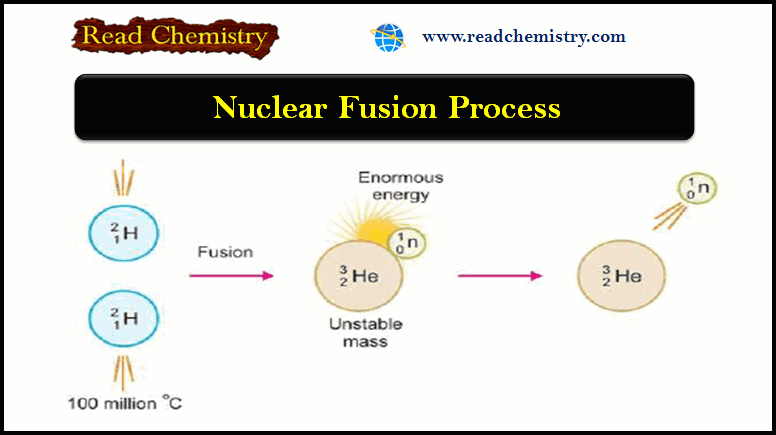
Nuclear Fusion: Definition, Occurrence, Examples, Applications
– In this subject, we will discuss the Nuclear Fusion Process ( Definition, Occurrence, Examples, Applications) Nuclear Fusion Process –…
Read More » -
Organic Chemistry
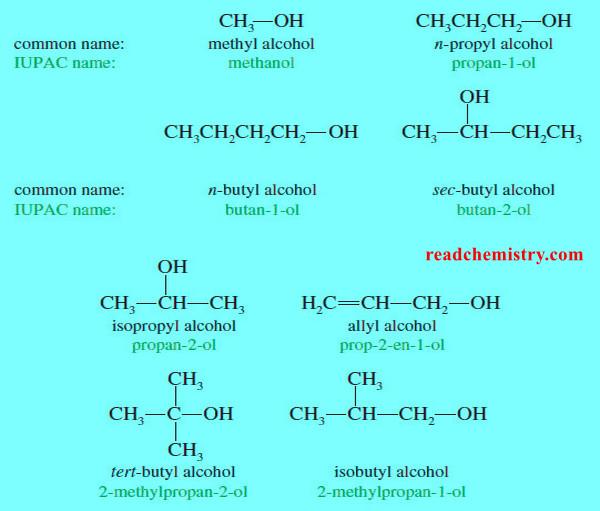
Nomenclature of Alcohols and Phenols
In this subject we will talk about Nomenclature of Alcohols and Phenols (1) Nomenclature of Alcohols: IUPAC Names – The…
Read More » -
Analytical Chemistry
Some Terms Used in Volumetric Titration
Some Terms Used in Volumetric Titration – A standard solution (or a standard titrant) is a reagent of known concentration…
Read More » -
Organic Chemistry
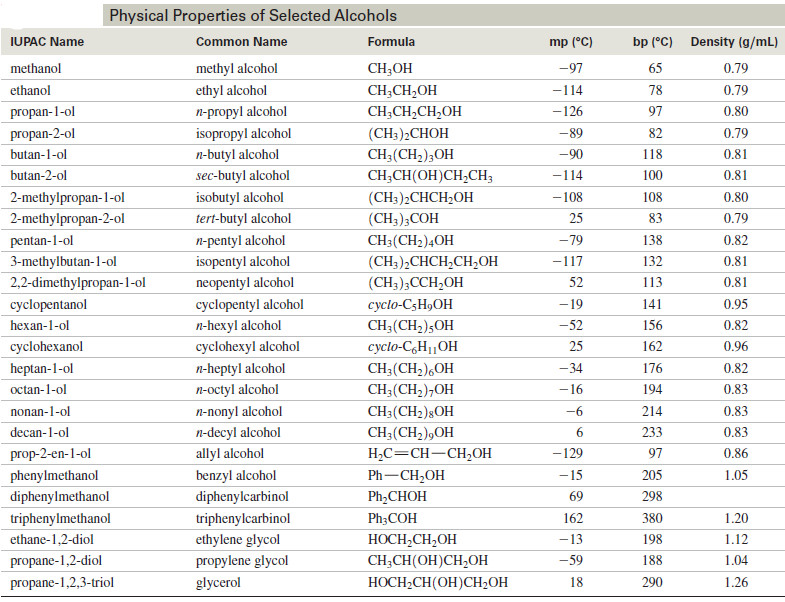
Physical Properties of Alcohols
We will discuss here Physical Properties of Alcohols: (A) Boiling Points of Alcohols (B) Solubility Properties of Alcohols Physical Properties…
Read More » -
Organic Chemistry
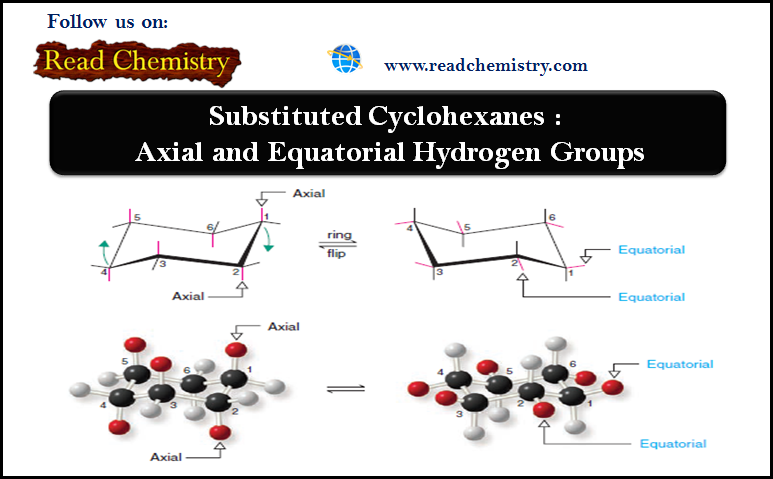
Cyclohexane: Axial and Equatorial Bonds in Cyclohexane
– In this subject, we will discuss the Substituted Cyclohexane: Axial and Equatorial Hydrogen Groups Substituted Cyclohexane: Axial and Equatorial…
Read More »
-
Organic Chemistry
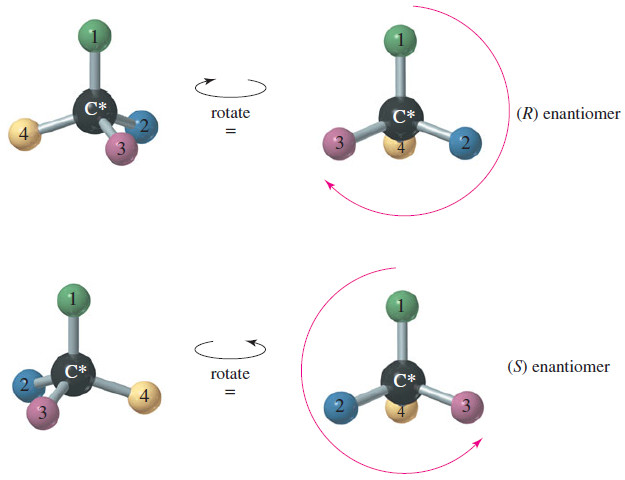
(R) and (S) of Asymmetric Carbon Atoms
(R) and (S) Nomenclature of Asymmetric Carbon Atoms – Alanine is one of the amino…
Read More » -
-
-
-
-
-
-
-
-
-
-
Physical Chemistry
Autocatalysis, Catalytic poisoning and Negative Catalysis
– In this topic, we will discuss Autocatalysis, Catalytic poisoning and Negative Catalysis. Catalytic poisoning…
Read More » -
-
-
-
-
-
-
-
-
-
-
General Chemistry
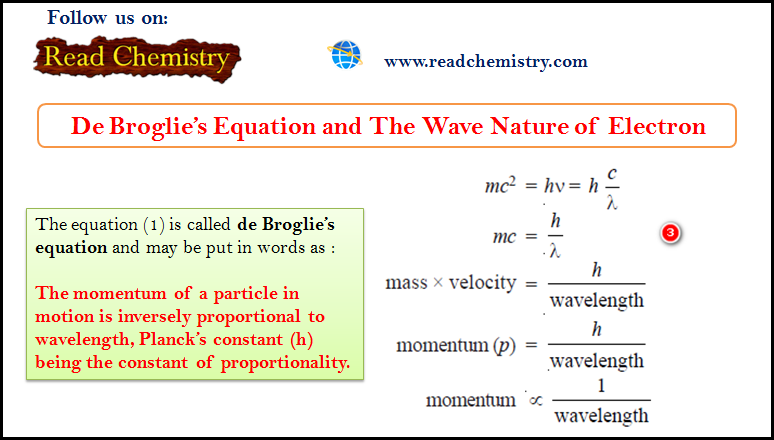
De Broglie Equation – The Wave Nature of Electron
De Broglie Equation – de Broglie had arrived at his hypothesis (de Broglie equation) with…
Read More » -
-
-
-
-
-
-
-
-
-
-
Analytical Chemistry
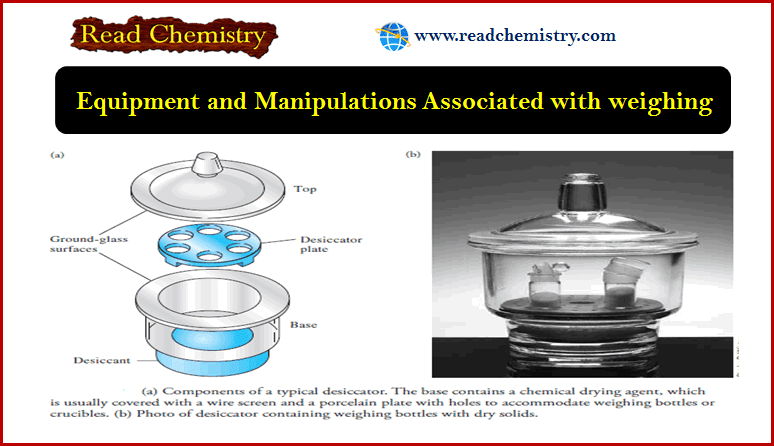
Precipitation Gravimetry
– In this topic, we will discuss the Precipitation Gravimetry as an important one of…
Read More » -
-
-
-
-
-
-
-
-
-
-
Online MCQ
First law of thermodynamics – MCQ online test
Online MCQ test on First law of thermodynamics – In this topic we offer you,…
Read More » -
-
-
-
-
-
-
-
Free book
Physical Chemistry book , 3rd edition by Robert G. Mortimer
– In this subject, we will discuss free download of Physical Chemistry book, 3rd edition…
Read More » -
-
-
-
-