Filtration and Ignition of Solids
– In this subject, we will discuss the Filtration and Ignition of Solids.
– Several techniques and experimental arrangements allow solids to be filtered and ignited with minimal contamination and error.
(1) Apparatus for Filtration of Solids
(a) Simple Crucibles
– Simple crucibles serve only as containers.
– Porcelain, aluminum oxide, silica, and platinum crucibles maintain constant mass—within the limits of experimental error— and are used principally to convert a precipitate into a suitable weighing form.
– The solid is first collected on a filter paper.
– The filter and contents are then transferred to a weighed crucible, and the paper is ignited.
– Simple crucibles of nickel, iron, silver, and gold are used as containers for the high-temperature fusion of samples that are not soluble in aqueous reagents.
– Attack by both the atmosphere and the contents may cause these crucibles to suffer mass changes.
– Moreover, such an attack will contaminate the sample with species derived from the crucible.
– The crucible whose products will offer the least interference in subsequent steps of the analysis should be used.
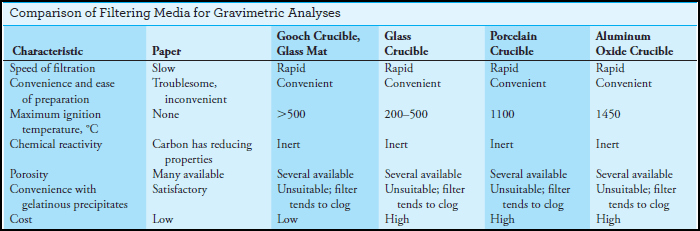
(b) Filtering Crucibles
– Filtering crucibles serve not only as containers but also as filters.
– A vacuum is used to hasten the filtration.
– A tight seal between the crucible and filtering flask is made with any of several types of rubber adaptors.
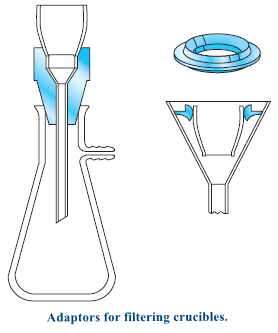
– Collection of a precipitate with a filtering crucible is frequently less time-consuming than with paper.
(1) Sintered-glass crucibles
– It is also called fritted-glass crucibles.
– They are manufactured in fine, medium, and coarse porosities (marked f, m, and c).
– The upper-temperature limit for a sintered-glass crucible is usually about 200°C.
– Filtering crucibles made entirely of quartz can tolerate substantially higher temperatures without damage.
– The same is true for crucibles with unglazed porcelain or aluminum oxide frits.
– The latter are not as costly as quartz.
(2) A Gooch crucible
– It has a perforated bottom that supports a fibrous mat.
– Asbestos was at one time the filtering medium of choice for a Gooch crucible.
– However, current regulations on the use of asbestos have virtually eliminated its use.
– Small circles of glass matting have now replaced asbestos.
– They are used in pairs to protect against breaking during the filtration.
– Glass mats can tolerate temperatures over 500°C and are substantially less hygroscopic than asbestos.
(c) Filter Paper
– Paper is an important filtering medium.
– Ashless paper is manufactured from cellulose fibers that have been treated with hydrochloric and hydrofluoric acids to remove metallic impurities and silica; ammonia is then used to neutralize the acids.
– The residual ammonium salts in many filter papers may be sufficient to affect the analysis for nitrogen by the Kjeldahl method.
– All papers tend to pick up moisture from the atmosphere, and ashless paper is no exception.
– It is thus necessary to destroy the paper by ignition if the precipitate collected on it is to be weighed.
– Typically, 9- or 11-cm circles of ashless paper leave a residue that weighs less than 0.1 mg, which is negligible under most circumstances.
– Ashless paper can be obtained in several porosities.
– Gelatinous precipitates, such as hydrous iron(III) oxide, clog the pores of any filtering medium.
– A coarse-porosity ashless paper is most effective for filtering such solids, but even with such paper, clogging occurs.
– This problem can be minimized by mixing a dispersion of ashless filter paper with the precipitate before filtration.
– Filter paper pulp is available in tablet form from chemical suppliers.
– If no commercial pulp is available, it can be prepared by treating a piece of ashless paper with concentrated hydrochloric acid and washing the disintegrated mass free of acid.
– This Table summarizes the characteristics of common filtering media. None satisfies all requirements.
(d) Heating Equipment
– Many precipitates can be weighed directly after being brought to constant mass in a low-temperature drying oven.
– Such an oven is electrically heated and capable of maintaining a constant temperature to within 1°C (or better).
– The maximum attainable temperature ranges from 140°C to 260°C, depending on the make and model.
– For many precipitates, 110°C is a satisfactory drying temperature.
– The efficiency of a drying oven is greatly increased by the forced circulation of air.
– The passage of predried air through an oven designed to operate under a partial vacuum represents an additional improvement.
(1) Microwave laboratory ovens
– They are currently quite popular, and where applicable, they greatly shorten drying cycles.
– For example, slurry samples that require 12 to 16 hours for drying in a conventional oven are reported to be dried within 5 to 6 minutes in a microwave oven.
– The time needed to dry silver chloride, calcium oxalate, and barium sulfate precipitates for gravimetric analysis is also shortened significantly.
(2) An ordinary heat lamp
– It can be used to dry a precipitate that has been collected on ashless paper and to char the paper as well.
– The process is conveniently completed by ignition at an elevated temperature in a muffle furnace.
(3) Burners
– They are convenient sources of intense heat.
– The maximum attainable temperature depends on the design of the burner and the combustion properties of the fuel.
– Of the three common laboratory burners, the Maker burner provides the highest temperatures, followed by the Tirrill and Bunsen types.
(4) A heavy-duty electric furnace (muffle furnace)
– It is capable of maintaining controlled temperatures of 1100°C or higher.
– Long-handled tongs and heat-resistant gloves are needed for protection when transferring objects to or from such a furnace.
(2) Filtration and Ignition of Precipitates
(a) Preparation of Crucibles
– A crucible used to convert a precipitate to a form suitable for weighing must maintain— within the limits of experimental error—a constant mass throughout drying or ignition.
– The crucible is first cleaned thoroughly (filtering crucibles are conveniently cleaned by backwashing on a filtration train) and then subjected to the same regimen of heating and cooling as that required for the precipitate.
– This process is repeated until constant mass has been achieved, that is, until consecutive weighings differ by 0.3 mg or less.
(b) Filtration and Washing Precipitates
– The steps in filtering an analytical precipitate are decantation, washing, and transfer.
– In decantation, as much supernatant liquid as possible is passed through the filter while the precipitated solid is kept essentially undisturbed in the beaker where it was formed.
– This procedure speeds up the overall filtration rate by delaying the time at which the pores of the filtering medium become clogged with precipitate.
– A stirring rod is used to direct the flow of the decanted liquid (Figure a).

– When flow ceases, the drop of liquid at the end of the pouring spout is collected with the stirring rod and returned to the beaker.
– Wash liquid is next added to the beaker and thoroughly mixed with precipitate.
– The solid is allowed to settle, and then this liquid is also decanted through the filter.
Several such washings may be required, depending on the precipitate. Most washing should be carried out before the bulk of the solid is transferred.
– This technique results in a more thoroughly washed precipitate and a more rapid filtration.
– The transfer process is illustrated in Figure (b).
– The bulk of the precipitate is moved from the beaker to the filter by directed streams of wash liquid.
– As in decantation and washing, a stirring rod provides direction for the flow of material to the filtering medium.
– The last traces of precipitate that cling to the inside of the beaker are dislodged with a rubber policeman, which is a small section of rubber tubing that has been crimped on one end.
– The open end of the tubing is fitted onto the end of a stirring rod and is wetted with wash liquid before use.
– Any solid collected with it is combined with the main portion of the filter.
– Small pieces of ashless paper can be used to wipe the last traces of hydrous oxide precipitates from the wall of the beaker.
– These papers are ignited along with the paper that holds the bulk of the precipitate.
– Many precipitates possess the exasperating property of creeping or spreading over a wetted surface against the force of gravity.
– Filters are never filled to more than three-quarters of capacity to prevent the possible loss of precipitate through creeping.
– The addition of a small amount of nonionic detergent, such as Triton X-100, to the supernatant liquid or wash liquid can help minimize creeping.
– A gelatinous precipitate must be completely washed before it is allowed to dry.
– These precipitates shrink and develop cracks as they dry.
– Further additions of wash liquid simply pass through these cracks and accomplish little or no washing.
(3) Directions for Filtration and Ignition Precipitates
(a) Preparation of a Filter Paper
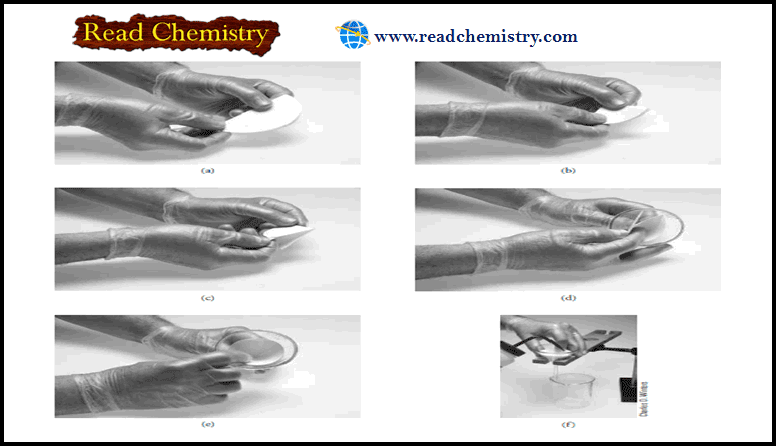
– Figure (1) shows the sequence for folding and seating a filter paper in a 60-deg funnel as follows:
(a) Fold the paper exactly in half and crease it firmly.
(b) Fold the paper a second time.
(c) Tear off one of the corners on a line parallel to the second fold.
(d) Open the untorn half of the folded paper to form a cone.
(e) Seat the cone firmly into the funnel.
(f) Moisten the paper slightly and gently pat the paper into place.
– There will be no leakage of air between the funnel and a properly seated cone.
– In addition, the stem of the funnel will be filled with an unbroken column of liquid.
(b) Transferring Paper and Precipitate to a Crucible
– After filtration and washing have been completed, the filter and its contents must be transferred from the funnel to a crucible that has been brought to constant mass.
– Ashless paper has very low wet strength and must be handled with care during the transfer.
– The danger of tearing is lessened considerably if the paper is allowed to dry somewhat before it is removed from the funnel.
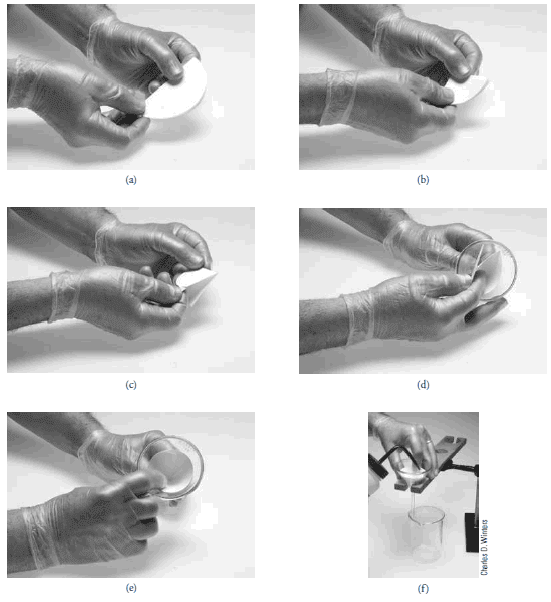
– Figure (2) Shows Transferring a filter paper and precipitate from a funnel to a crucible as follows:
(a) Pull the triple-thick portion of the cone to the opposite side of the funnel.
(b) Remove the filter cone from the funnel, and flatten the cone along its upper edge.
(c) Fold the corners inward.
(d) Fold the top edge of the cone toward the tip to enclose the precipitate in the paper.

(e) Gently ease the folded paper and its contents into the crucible.
(c) Ashing Filter Papers
– If a heat lamp is used, the crucible is placed on a clean, nonreactive surface, such as a wire screen covered with aluminum foil.
– The lamp is then positioned about 1 cm above the rim of the crucible and turned on.
– Charring takes place without further attention.
– The process is considerably accelerated if the paper is moistened with no more than one drop of concentrated ammonium nitrate solution.
– The residual carbon is eliminated with a burner, as described in the next paragraph.
– Considerably more attention must be paid if a burner is used to ash a filter paper because the burner produces much higher temperatures than a heat lamp.
– Thus, mechanical loss of precipitate may occur if moisture is expelled too rapidly in the initial stages of heating or if the paper bursts into flame.
– Also, partial reduction of some precipitates can occur through a reaction with the hot carbon of the charring paper.
– This reduction is a serious problem if reoxidation following ashing is inconvenient.
– These difficulties can be minimized by positioning the crucible as illustrated in Figure (3).
– The tilted position allows for easy access to air.
– A clean crucible cover should be kept handy to extinguish any flame.

– Heating should begin with a small flame.
– The temperature is gradually increased as moisture evolves and the paper begins to char.
– The amount of smoke given off indicates the intensity of heating that can be tolerated. Thin wisps are normal.
– A significant increase in smoke indicates that the paper is about to flash and that heating should be temporarily discontinued.
– Any flame should be immediately extinguished with a crucible cover. (The cover may become discolored from the condensation of carbonaceous products.
– These products must ultimately be removed from the cover by ignition to confirm the absence of entrained particles of precipitate.)
– When no further smoking can be detected, heating is increased to eliminate the residual carbon.
– Strong heating, as necessary, can then be undertaken.
– This sequence usually precedes the final ignition of a precipitate in a muffle furnace, where a reducing atmosphere is equally undesirable.
(d) Using Filtering Crucibles
– A vacuum filtration train (Figure 4) is used when a filtering crucible can be used instead of paper.

– The trap isolates the filter flask from the source of the vacuum.
(4) Rules for Manipulating Heated Objects
– Careful adherence to the following rules will minimize the possibility of accidental loss of a precipitate:
(1) Practice unfamiliar manipulations before putting them to use.
(2) Never place a heated object on the benchtop. Instead, place it on a wire gauze or a heat-resistant ceramic plate.
(3) Allow a crucible that has been subjected to the full flame of a burner or to a muffle furnace to cool momentarily (on a wire gauze or ceramic plate) before transferring it to the desiccator.
(4) Keep the tongs and forceps used to handle heated objects scrupulously clean. In particular, do not allow the tips to touch the benchtop.
Reference: Fundamentals of analytical chemistry / Douglas A. Skoog, Donald M. West, F. James Holler, Stanley R. Crouch. (ninth edition) , 2014 . USA