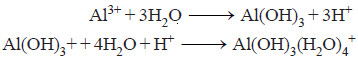Applications of Colloids
– In this topic, we will discuss The Applications of Colloids.
Applications of Colloids
– Colloids play an important role in our daily life and industry.
– A knowledge of colloid chemistry is essential to understand some of the various natural phenomena around us.
– Colloids make up some of our modern products.
– A few of the important applications of colloids are listed below.
(1) Foods
– Many of our foods are colloidal in nature.
– Milk is an emulsion of butterfat in water protected by a protein, casein.
– Salad dressing, gelatin deserts, fruit jellies and whipped cream are other examples.
– Ice cream is a dispersion of ice in cream.
– Bread is a dispersion of air in baked dough.
(2) Medicines
– Colloidal medicines being finely divided, are more effective and are easily absorbed in our system.
– Halibut-liver oil and cod-liver that we take are, in fact, the emulsions of the respective oils in water.
– Many ointments for application to skin consist of physiologically active components dissolved in oil and made into an emulsion with water.
– Antibiotics such as penicillin and streptomycin are produced in colloidal form suitable for injections.
(3) Non-drip or thixotropic paints
– All paints are colloidal dispersions of solid pigments in a liquid medium.
– The modern nondrip or thixotropic paints also contain long-chain polymers.
– At rest, the chains of molecules are coiled and entrap much dispersion medium. Thus the paint is a semisolid gel structure.
– When shearing stress is applied with a paint brush, the coiled molecules straighten and the entrapped medium is released.
– As soon as the brush is removed, the liquid paint reverts to the semisolid form. This renders the paint ‘non-drip’.
(4) Electrical precipitation of smoke
– The smoke coming from industrial plants is a colloidal dispersion of solid particles (carbon, arsenic compounds, cement dust) in air. It is a nuisance and pollutes the atmosphere.
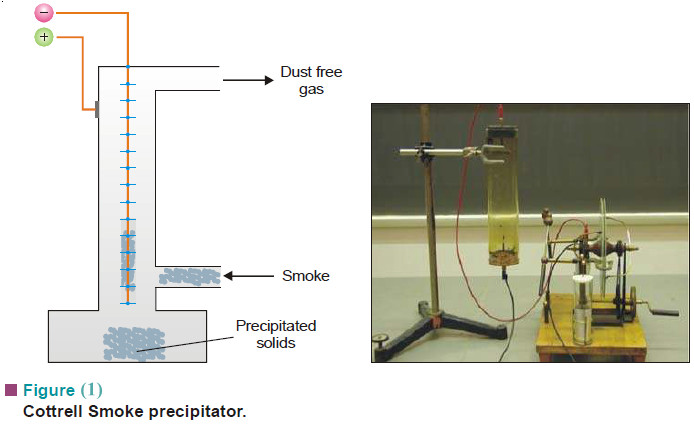
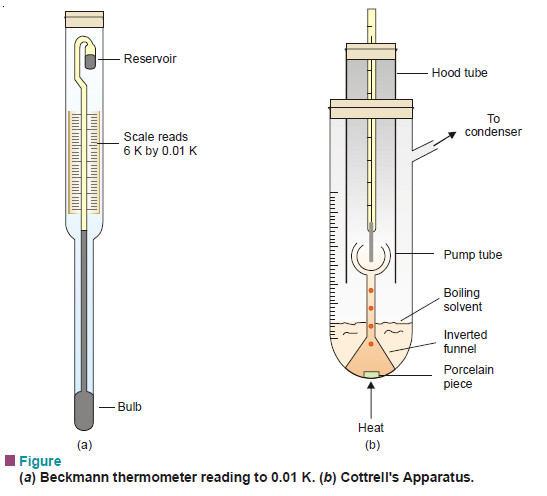
– Therefore, before allowing the smoke to escape into air, it is treated by Cottrell Precipitator (See Fig. 1).
– The smoke is let past a series of sharp points charged to a high potential (20,000 to 70,000 V).
– The points discharge high velocity electrons that ionise molecules in air.
– Smoke particles adsorb these positive ions and become charged.
– The charged particles are attracted to the oppositely charged electrodes and get precipitated.
– The gases that leave the Cottrell precipitator are thus freed from smoke.
– In addition, valuable materials may be recovered from the precipitated smoke.
– For example, arsenic oxide is mainly recovered from the smelter smoke by this method.
(5) Clarification of Municipal water
– The municipal water obtained from natural sources often contains colloidal particles.
– The process of coagulation is used to remove these.
– The sol particles carry a negative charge.
– When aluminium sulphate (alum) is added to water, a gelatinous precipitate of hydrated aluminium hydroxide (floc) is formed,
– The positively charged floc attracts to it negative sol particles which are coagulated.
– The floc along with the suspended matter comes down, leaving the water clear.
(6) Formation of Delta
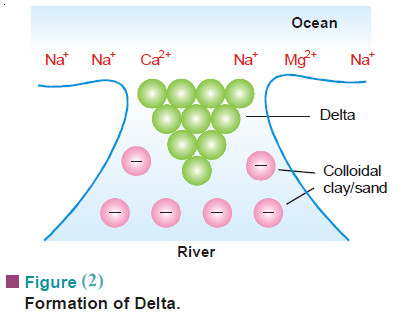
– The river water contains colloidal particles of sand and clay which carry negative charge.
– The sea water, on the other hand, contains positive ions such as Na+, Mg2+, Ca2+.
– As the river water meets sea water, these ions discharge the sand or clay particles which are precipitated as delta.
(7) Artificial Kidney machine
– The human kidneys purify the blood by dialysis through natural membranes.
– The toxic waste products such as urea and uric acid pass through the membranes, while colloidal-sized particles of blood proteins (haemoglobin) are retained.
– Kidney failure, therefore, leads to death due to accumulation of poisonous waste products in blood .
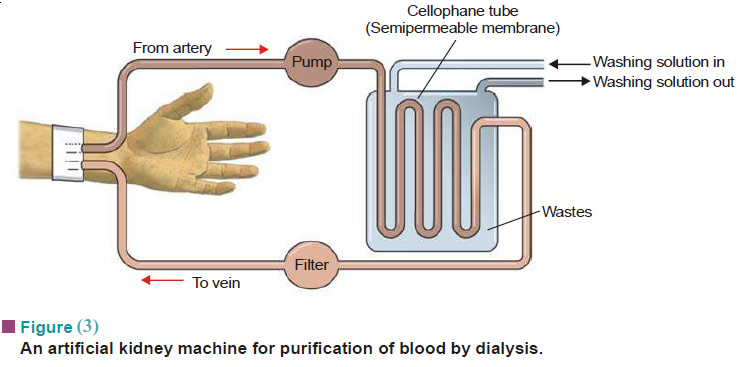
– Now-a-days, the patient’s blood can be cleansed by shunting it into an ‘artificial kidney machine’.
– Here the impure blood is made to pass through a series of cellophane tubes surrounded by a washing solution in water.
– The toxic waste chemicals (urea, uric acid) diffuse across the tube walls into the washing solution.
– The purified blood is returned to the patient.
– The use of artificial kidney machine saves the life of thousands of persons each year.
(8) Adsorption indicators
– Adsorption indicators function by preferential adsorption of ions onto sol particles.
– Fluorescein (Na+Fl) is an example of adsorption indicator which is used for the titration of sodium chloride solution against silver nitrate solution.
– When silver nitrate solution is run into a solution of sodium chloride containing a little fluorescein, a white precipitate of silver chloride is first formed.
– At the end-point, the white precipitate turns sharply pink.
Explanation
– The indicator fluorescein is a dye (Na+Fl–) which gives coloured anion Fl– in aqueous solution.
– The white precipitate of silver chloride formed by running AgNO3 solution into NaCl solution is partially colloidal in nature.
(a) Before the end-point
-Before the end-point, Cl– ions are in excess.
– The AgCl sol particles adsorb these ions and become negatively charged.
– The negative AgCl/Cl– particles cannot adsorb the coloured fluorescein anions (Fl–) due to electrostatic repulsion.
– Thus the precipitate remains white.
(b) After the end-point
– After the end-point, Ag+ ions become in excess.
– AgCl sol particles adsorb these and acquire positive charge.
– The positive AgCl/Ag+ particles now attract the coloured fluorescein anions (Fl–) and turn rose-red.
– Thus the end-point is marked by white precipitate changing to pink.
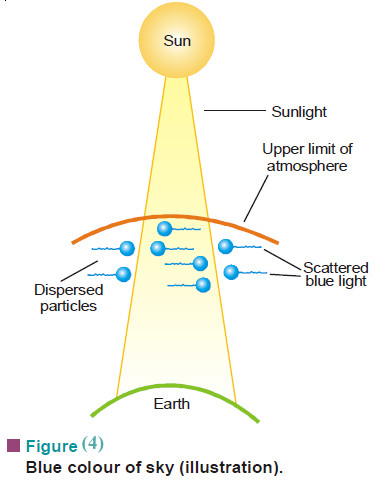
(9) Blue colour of the sky
– This is an application of Tyndall effect.
– The upper atmosphere contains colloidal dust or ice particles dispersed in air.
– As the sun rays enter the atmosphere (Fig.4) these strike the colloidal particles.
– The particles absorb sunlight and scatter light of blue colour (4600–5100Å).
– The light that is incident at earth’s surface is considerably reddened due to the removal of most of the blue light in the upper atmosphere.
– please, comment on Applications of Colloids topic.