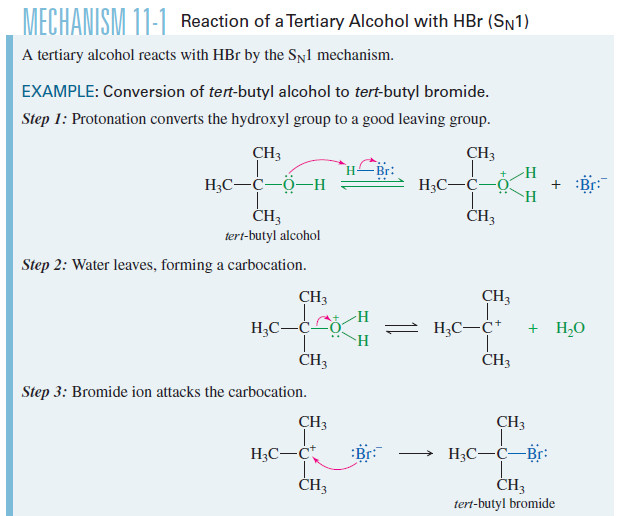
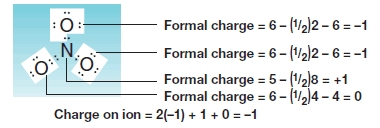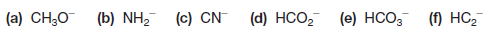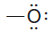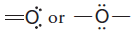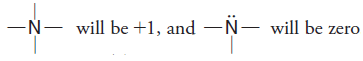Formal Charge: Definition, Formula, Calculation, Examples
– In this subject, we will discuss the Formal Charge: Definition, Formula, Calculation, Examples
Formal Charge and How To Calculate
– Many Lewis structures are incomplete until we decide whether any of their atoms have a formal charge.
– Calculating the formal charge on an atom in a Lewis structure is simply a bookkeeping method for its valence electrons.
– First, we examine each atom and, using the periodic table, we determine how many valence electrons it would have if it were an atom not bonded to any other atoms.
– This is equal to the group number of the atom in the periodic table.
– For hydrogen, this number equals 1, for carbon it equals 4, for nitrogen it equals 5, and for oxygen, it equals 6.
– Next, we examine the atom in the Lewis structure and we assign the valence electrons in the following way:
– We assign to each atom half of the electrons it is sharing with another atom and all of its unshared (lone) electron pairs.
– Then we do the following calculation for the atom:
– Formal charge = number of valence electrons – 1/2 number of shared electrons – number of unshared electrons.
or
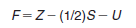
– It is important to note, too, that the arithmetic sum of all the formal charges in a molecule or ion will equal the overall charge on the molecule or ion.
Examples of Calculating Formal Charge
– Let us consider several examples showing how this is done.
The Ammonium Ion (NH4+)
– As we see below, the ammonium ion has no unshared electron pairs.
– We divide all of the electrons in bonds equally between the atoms that share them. Thus, each hydrogen is assigned one electron.
– We subtract this from one (the number of valence electrons in a hydrogen atom) to give each hydrogen atom a formal charge of zero.
– The nitrogen atom is assigned four electrons (one from each bond).
– We subtract four from five (the number of valence electrons in a nitrogen atom) to give the nitrogen a formal charge of +1.
The Nitrate Ion (NO3–)
– Let us next consider the nitrate ion (NO3–), an ion that has oxygen atoms with unshared electron pairs.
– Here we find that the nitrogen atom has a formal charge of +1, that two oxygen atoms have formal charges of -1, and that one oxygen has a formal charge equal to 0.
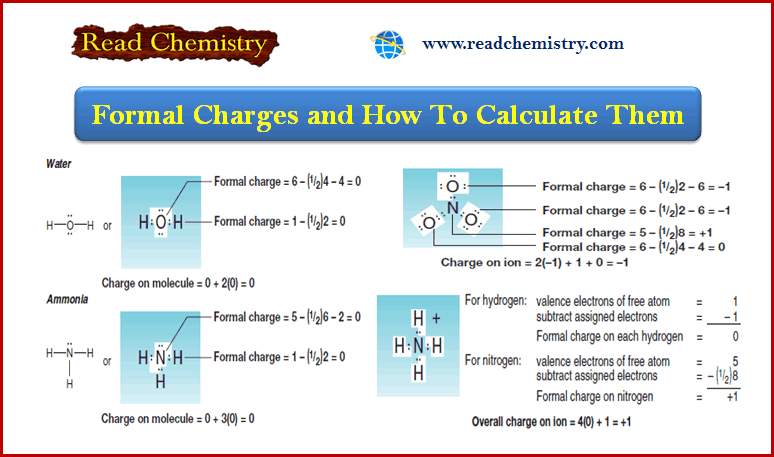
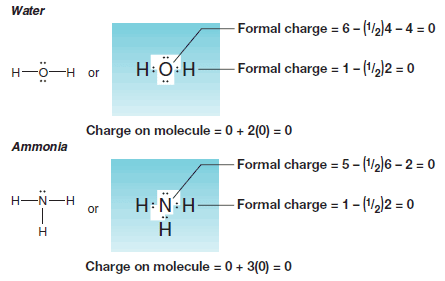
Water and Ammonia
– The sum of the formal charges on each atom making up a molecule must be zero.
– Consider the following examples:
Practice Problem(1): Write a Lewis structure for each of the following negative ions, and assign the formal negative charge to the correct atom:
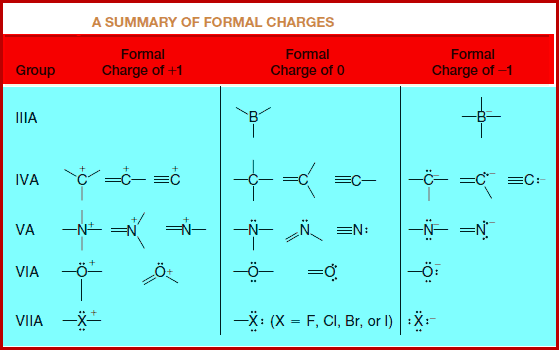
A Summary of Formal Charge
– With this background, it should now be clear that each time an oxygen atom of the type:
appears in a molecule or ion, it will have a formal charge of -1, and each time an oxygen atom of the type :
appears, it will have a formal charge of 0.
– Similarly,
– These and other common structures are summarized in this Table:
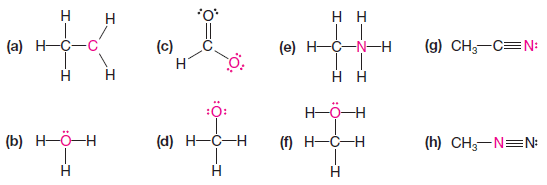
Practice Problem(2): Assign the proper formal charge to the colored atom in each of the following structures:
Reference: Organic chemistry / T.W. Graham Solomons, Craig B.Fryhle, Scott A. Snyder, / ( eleventh edition) / 2014.