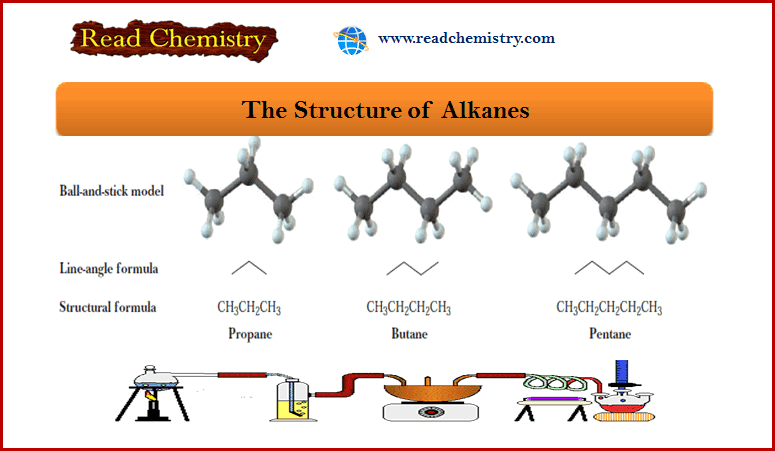
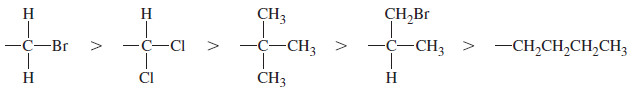(R) and (S) of Asymmetric Carbon Atoms
(R) and (S) Nomenclature of Asymmetric Carbon Atoms
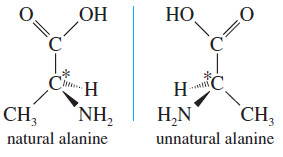
– Alanine is one of the amino acids found in common proteins.
– Alanine has an asymmetric carbon atom, and it exists in two enantiomeric forms.
– These mirror images are different, and this difference is reflected in their biochemistry.
– Only the enantiomer on the left can be metabolized by the usual enzyme; the one on the right is not recognized as a useful amino acid.
– Both are named alanine, however, or 2 aminopropanoic acid in the IUPAC system.
– We need a simple way to distinguish between enantiomers and to give each of them a unique name.
– The difference between the two enantiomers of alanine lies in the three-dimensional arrangement of the four groups around the asymmetric carbon atom.
– Any asymmetric carbon has two possible (mirror-image) spatial arrangements, which we call configurations.
– The alanine enantiomers represent the two possible arrangements of its four groups around the asymmetric carbon atom. If we can name the two configurations of any asymmetric carbon atom, then we have a way of specifying and naming the enantiomers of alanine or any other chiral compound.
The Cahn–Ingold–Prelog convention
– The Cahn–Ingold–Prelog convention is the most widely accepted system for naming the configurations of chirality centers.
– Each asymmetric carbon atom is assigned a letter (R) or (S) based on its three-dimensional configuration.
– To determine the name, we follow a two-step procedure that assigns “priorities” to the four substituents and then assigns the name based on the relative positions of these substituents.
– Here is the procedure:
(1) Assign a relative “priority” to each group bonded to the asymmetric carbon.
We speak of group 1 as having the highest priority, group 2 second, group 3 third, and group 4 as having the lowest priority.
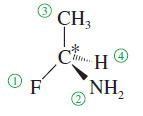
(a) Look at the first atom of the group—the atom bonded to the asymmetric carbon. Atoms with higher atomic numbers receive higher priorities.
– For example, if the four groups bonded to an asymmetric carbon atom were H, and F, the fluorine atom (atomic number 9) would have the highest priority, followed by the nitrogen atom of the NH2 group (atomic number 7), then by the carbon atom of the methyl group (atomic number 6).
– Note that we look only at the atomic number of the atom directly attached to the asymmetric carbon, not the entire group. Hydrogen would have the lowest priority.
– With different isotopes of the same element, the heavier isotopes have higher priorities. For example, tritium receives a higher priority than deuterium followed by hydrogen
– Examples of priority for atoms bonded to an asymmetric carbon:
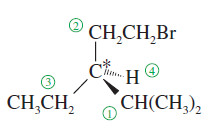
(b) In case of ties, use the next atoms along the chain of each group as tiebreakers.
– For example, we assign a higher priority to isopropyl -CH(CH3)2 than to ethyl -CH2CH3 or bromoethyl -CH2CH2Br
– The first carbon in the isopropyl group is bonded to two carbons, while the first carbon in the ethyl group (or the bromoethyl group) is bonded to only one carbon.
– An ethyl group and a -CH2CH2Br have identical first atoms and second atoms, but the bromine atom in the third position gives -CH2CH2Br a higher priority than -CH2CH3
– One high-priority atom takes priority over any number of lower-priority atoms.
Examples:
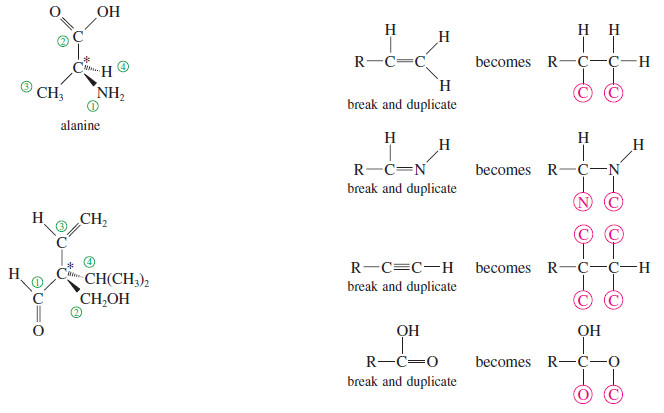
(c) Treat double and triple bonds as if each were a bond to a separate atom.
For this method, imagine that each pi bond is broken and the atoms at both ends duplicated. Note that when you break a bond, you always add two imaginary atoms. (Imaginary atoms are circled below.)
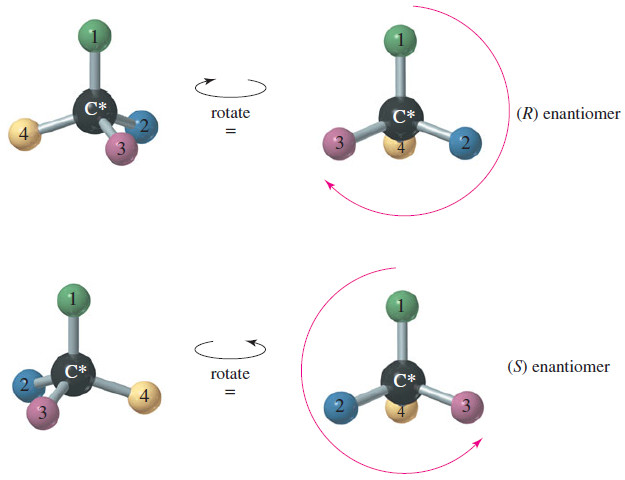
2. Using a three-dimensional drawing or a model, put the fourth-priority group away from you and view the molecule with the first, second, and third priority groups radiating toward you like the spokes of a steering wheel. Draw an arrow from the first-priority group, through the second, to the third.
If the arrow points clockwise, the asymmetric carbon atom is called (R) (Latin, rectus, “upright”). If the arrow points counterclockwise, the chiral carbon atom is called (S) (Latin, sinister, “left”).
– Alternatively, you can draw the arrow and imagine turning a car’s steering wheel in that direction. If the car would go to the left, the asymmetric carbon atom is designated (S). If the car would go to the right, the asymmetric carbon atom is designated (R).
– Let’s use the enantiomers of alanine as an example.
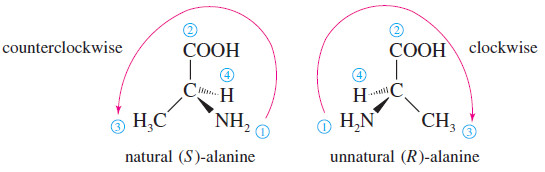
– The naturally occurring enantiomer is the one on the left, determined to have the (S) configuration.
– Of the four atoms attached to the asymmetric carbon in alanine, nitrogen has the largest atomic number, giving it the highest priority.
– Next is the carbon atom, since it is bonded to oxygen atoms. Third is the methyl group, followed by the hydrogen atom.
– When we position the natural enantiomer with its hydrogen atom pointing away from us, the arrow from to to points counterclockwise.
– Thus, the naturally occurring enantiomer of alanine has the (S) configuration.
– Make models of these enantiomers to illustrate how they are named (R) and (S).
Solved Problems on (R) and (S) enantiomers
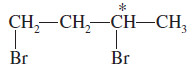
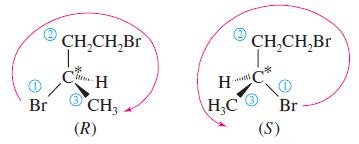
Problem (1): Draw the enantiomers of 1,3-dibromobutane and label them as (R) and (S). (Making a model is particularly helpful for this type of problem.)
Solution:
– The third carbon atom in 1,3-dibromobutane is asymmetric. The bromine atom receives first priority, the (-CH2CH2Br) group second priority, the methyl group third, and the hydrogen fourth.
– The following mirror images are drawn with the hydrogen atom back, ready to assign (R) or (S) as shown.
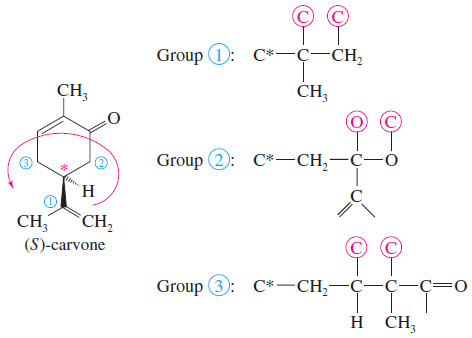
Problem (2): The structure of one of the enantiomers of carvone is shown here. Find the asymmetric carbon atom, and determine whether it has the (R) or the (S) configuration.
Solution:
– The asymmetric carbon atom is one of the ring carbons, as indicated by the asterisk in the following structure.
– Although there are two -CH2– groups bonded to the carbon, they are different -CH2– groups.
– One is a -CH2-CO- a group, and the other is a -CH2-CH=C group.
– The groups are assigned priorities, and this is found to be the (S) enantiomer.