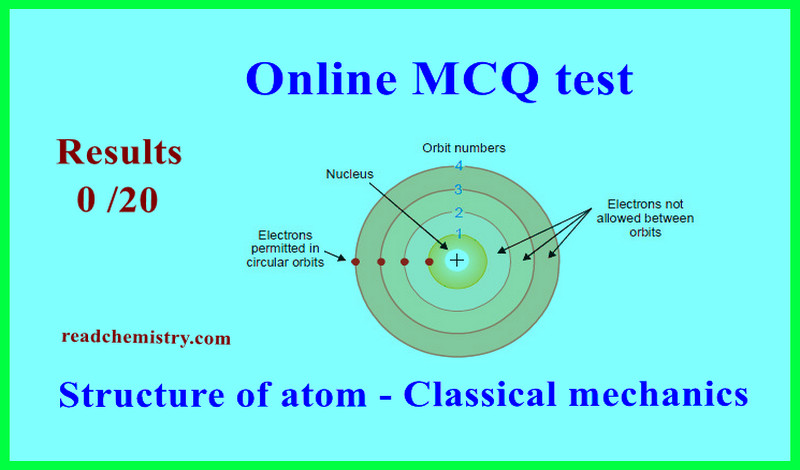
Structure of atom – Classical mechanics – Online MCQ test
Online MCQ test on Structure of atom – Classical mechanics
– In this topic we offer you, online MCQ test in the fundmental of Structure of atom – Classical mechanics .
– It is a simple test to measure the strength of your knowledge in the basics of understanding the topics of Structure of atom – Classical mechanics.
– The test consists of only (20) questions (multiple choice) you must answer all of them so that your final score appears automatically at the top of the topic.
– you will pass the Exam If get 70% or more.
– If you fail the test, you can retake the test again by reloading the page
– Let’s start the test, good luck.
Starting the test of Structure of atom – Classical mechanics
Results
Congratulation
Try again
#1. The charge to mass ratio (e/m) of positive particles.
#2. The Balmer series in the spectrum of hydrogen atom falls in ___
#3. Electromagnetic radiations with minimum wavelength is ____
#4. Atomic number of an element is equal to the number of _______ in the nucleus of the atom
#5. The spectrum of helium is expected to be similar to that of ___
#6. The ground state of an atom corresponds to a state of ________
#7. Out of the following pairs of elements which has the same number of electrons in the outer most energy level?
#8. The number of electrons in the outermost shell of Potassium (at. no. 19) is ___
#9. In the spectrum of hydrogen atom, the series which falls in ultraviolet region is
#10. An atom of Calcium (at. no. 20) contains _______ electrons in the third energy level.
#11. In Bohr’s model of atom, the angular momentum of an electron orbiting around the nucleus is given by the relation
#12. If (Z) is the number of proton and (A) the number of nucleons, then the number of neutrons is an atom is given by ___
#13. The effect of electric field on the spectra of atoms is called _____
#14. The energy of an electron in the first Bohr orbit for hydrogen is ____
#15. In a sodium atom (atomic number = 11 and mass number = 23), the number of neutrons is ___
#16. A sub atomic particle which has one unit mass and one unit positive charge is known as ___
#17. When the source emitting lines is placed in a strong magnetic field the spectral lines are split into its components. This effect is called ____
#18. The idea of stationary orbits was first given by ___
#19. The maximum number of electrons is the outermost orbit is ____
#20. Cathode rays are deflected by _____
Click on the word (Finish) to see the test result
MCQs test on Chemistry
– Our multiple choice questions and answers (MCQs) focus on all areas of Chemistry covering all chemistry topics.
– One can read MCQs on Chemistry here. These topics are chosen from a collection of the most authoritative and best reference books on Chemistry.
– One should spend 1 hour daily practicing these MCQs for 2-3 months to learn and assimilate Chemistry subject comprehensively.
– This way of systematic learning will prepare anyone easily for Chemistry – Class exams, contests, online tests, quizzes, MCQ-tests, viva-voce, interviews, and certifications.
– These MCQs are organized chapterwise and each Chapter is futher organized topicwise.
– Every MCQ set focuses on a specific topic of Chapter in Chemistry.
Who should Practice MCQ Chemistry ?
(1) Students who are preparing for various school tests such as unit-tests, term-tests, semester tests, half-yearly and yearly tests and examinations on Chemistry
(2) Students who are preparing for Online/Offline Tests/Contests in Chemistry.
(3) Students who wish to sharpen their knowledge of Chemistry.
(4) Students who are studying in CBSE, ICSE, IGCSE schools as well as various State Board schools.
(5) Students who are preparing for competitive examinations such as NTSE, Olympiad.
(6) Students who are preparing for Central board examinations in Chemistry namely CBSE, ICSE, and NIOS Board.
(7)Students who are preparing for State board examinations in Chemistry such as PUC, Intermediate exams, etc.
(8) Students who are preparing for engineering and medical entrance examinations like NEET, IIT JEE, AIIMS, JIPMER & SAT Exams in Chemistry.
Get more: MCQ online test in Chemistry by English
Get more: MCQ online test in Chemistry by Arabic