Tertiary structure of globular proteins
– In the last topic in biochemistry in our website, we talked about: Primary structure of Protein , Secondary Structure of Protein. now we will discuss Tertiary structure of globular proteins.
Tertiary structure of globular proteins
– The primary structure of a polypeptide chain determines its tertiary structure.
– “Tertiary” refers both to the folding of domains (the basic units of structure and function, see discussion below), and to the final arrangement of domains in the polypeptide.
– The structure of globular proteins in aqueous solution is compact, with a high-density (close packing) of the atoms in the core of the molecule.
– Hydrophobic side chains are buried in the interior, whereas hydro philic groups are generally found on the surface of the molecule.
A. Domains
– Domains are the fundamental functional and three-dimensional structural units of polypeptides.
– Polypeptide chains that are greater than 200 amino acids in length generally consist of two or more domains.
– The core of a domain is built from combinations of supersecondary structural elements (motifs).
– Folding of the peptide chain within a domain usually occurs independently of folding in other domains.
– Therefore, each domain has the characteristics of a small, compact globular protein that is structurally independent of the other domains in the polypeptide chain.
B. Interactions stabilizing tertiary structure
– The unique three-dimensional structure of each polypeptide is determined by its amino acid sequence.
– Interactions between the amino acid side chains guide the folding of the polypeptide to form a compact structure.
– The following four types of interactions cooperate in stabilizing the tertiary structures of globular proteins.
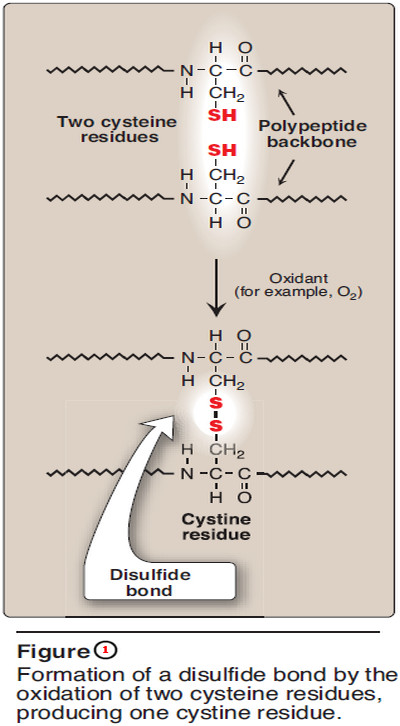
1. Disulfide bonds
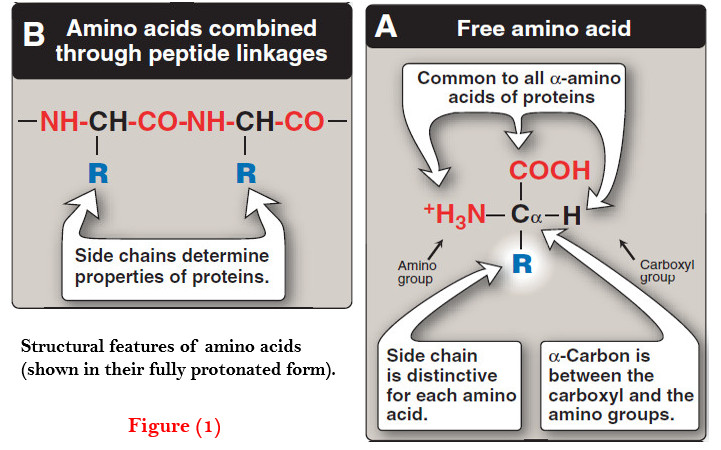
– A disulfide bond is a covalent linkage formed from the sulfhydryl group (–SH) of each of two cysteine residues, to produce a cystine residue (Figure 1).
– The two cysteines may be separated from each other by many amino acids in the primary sequence of a polypeptide, or may even be located on two different polypeptide chains; the folding of the polypeptide chain(s) brings the cysteine residues into proximity, and permits covalent bonding of their side chains.
– A disulfide bond contributes to the stability of the three-dimensional shape of the protein molecule, and prevents it from becoming denatured in the extracellular environment.
– For example, many disulfide bonds are found in proteins such as immunoglobulins that are secreted by cells.
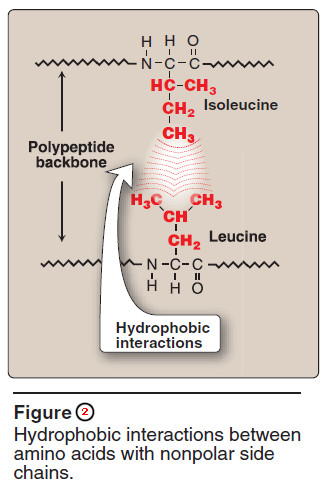
2. Hydrophobic interactions
– Amino acids with nonpolar side chains tend to be located in the interior of the polypeptide molecule, where they associate with other hydrophobic amino acids (Figure 2).
– In contrast, amino acids with polar or charged side chains tend to be located on the surface of the molecule in contact with the polar solvent.
– Note: Recall that proteins located in nonpolar (lipid) environments, such as a membrane, exhibit the reverse arrangement.
– In each case, a segregation of R-groups occurs that is energetically most favorable.
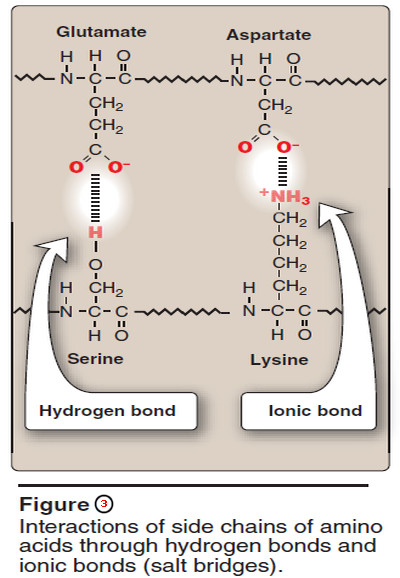
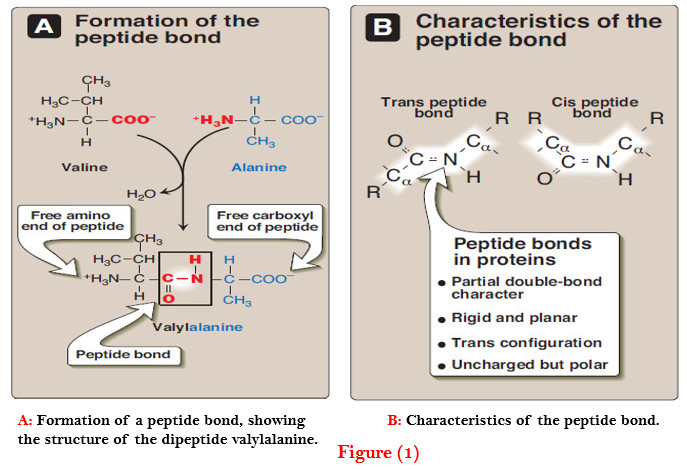
3. Hydrogen bonds
– Amino acid side chains containing oxygen- or nitrogen-bound hydrogen, such as in the alcohol groups of serine and threonine, can form hydrogen bonds with electron-rich atoms, such as the oxygen of a carboxyl group or carbonyl group of a peptide bond (Figure 3).
– Formation of hydrogen bonds between polar groups on the surface of proteins and the aqueous solvent enhances the solubility of the protein.
4. Ionic interactions
– Negatively charged groups, such as the carboxylate group (– COO–) in the side chain of aspartate or glutamate, can interact with positively charged groups, such as the amino group (– NH3+) in the side chain of lysine (see Figure 3).
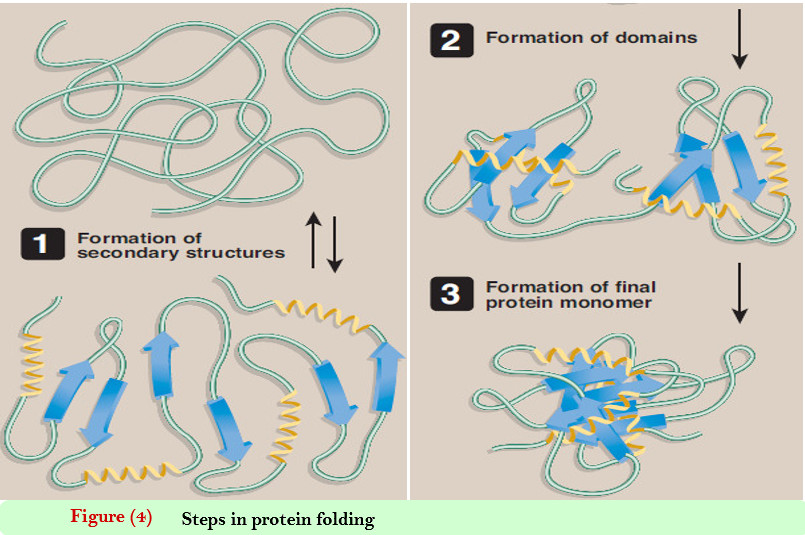
C. Protein folding
– Interactions between the side chains of amino acids determine how a long polypeptide chain folds into the intricate three-dimensional shape of the functional protein.
– Protein folding, which occurs within the cell in seconds to minutes, employs a shortcut through the maze of all folding possibilities.
– As a peptide folds, its amino acid side chains are attracted and repulsed according to their chemical properties.
– For example, positively and negatively charged side chains attract each other. Conversely, similarly charged side chains repel each other.
– In addition, interactions involving hydrogen bonds, hydrophobic interactions, and disulfide bonds all exert an influence on the folding process.
– This process of trial and error tests many, but not all, possible configurations, seeking a compromise in which attractions outweigh repulsions.
– This results in a correctly folded protein with a low-energy state (Figure 4).
D. Denaturation of proteins
– Protein denaturation results in the unfolding and disorganization of the protein’s secondary and tertiary structures, which are not accompanied by hydrolysis of peptide bonds.
– Denaturing agents include heat, organic solvents, mechanical mixing, strong acids or bases, detergents, and ions of heavy metals such as lead and mercury.
– Denaturation may, under ideal conditions, be reversible, in which case the protein refolds into its original native structure when the denaturing agent is removed. However, most proteins, once denatured, remain permanently disordered.
– Denatured proteins are often insoluble and, therefore, precipitate from solution.
E. Role of chaperones in protein folding
– It is generally accepted that the information needed for correct protein folding is contained in the primary structure of the polypeptide.
– Given that premise, it is difficult to explain why most proteins when denatured do not resume their native conformations under favorable environmental conditions.
– One answer to this problem is that a protein begins to fold in stages during its synthesis, rather than waiting for synthesis of the entire chain to be totally completed.
– This limits competing folding configurations made available by longer stretches of nascent peptide.
– In addition, a specialized group of proteins, named “chaperones,” are required for the proper folding of many species of proteins.
– The chaperones also known as “heat shock” proteins—interact with the polypeptide at various stages during the folding process.
– Some chaperones are important in keeping the protein unfolded until its synthesis is finished, or act as catalysts by increasing the rates of the final stages in the folding process.
– Others protect proteins as they fold so that their vulnerable, exposed regions do not become tangled in unproductive interactions.
References:
- Lehninger Principles of Biochemistry / David L. Nelson, Michael M. Cox/ 7th ed, 2017.
- Lippincott’s Illustrated Reviews: Biochemistry / Richard A. Harvey, Denise R. Ferrier/ 5th ed, 2011 / Lippincott Williams & Wilkins, USA.
- Harper’s Illustrated Biochemistry /Robert K. Murray, David A. Bender , Kathleen M. Botham / 28th, 2009/ McGraw-Hill Companies, Inc. USA.