Radioactivity: Detection and Measurement
– In this subject, we will discuss the Detection and measurement of Radioactivity.
Detection and measurement of Radioactivity
– Radioactivity can be detected and measured by several methods.
– The important ones used in modern practice are listed below.
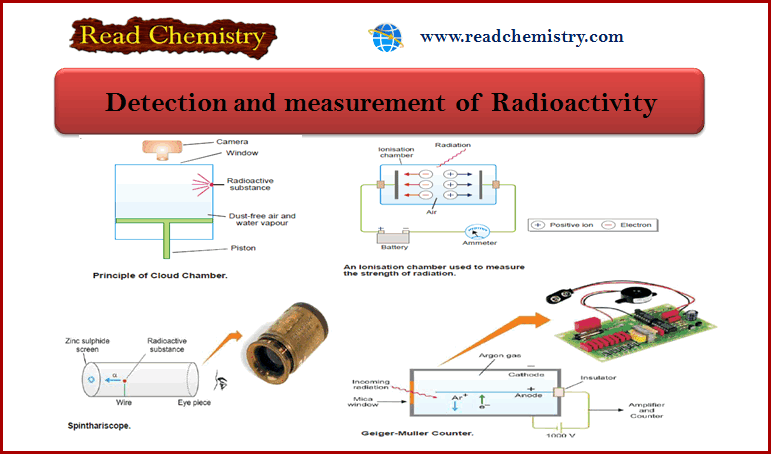
(1) Cloud Chamber
(2) Ionization Chamber
(3) Geiger-Muller Counter
(4) Scintillation Counter
(5) Film Badges
(1) Cloud Chamber
– This technique is used for detecting radioactivity.
– The chamber contains air saturated with water vapor.
– When the piston is lowered suddenly, the gas expands and is supercooled.
– As an α- or β-particle passes through the gas, ions are created along its path.
– These ions provide nuclei upon which droplets of water condense.
– The trail or cloud thus produced marks the track of the particle.
– The track can be seen through the window above and immediately photographed.
– Similarly, α- or β-particles form a trail of bubbles as they pass through liquid hydrogen.
– The bubble chamber method gives better photographs of the particle tracks.
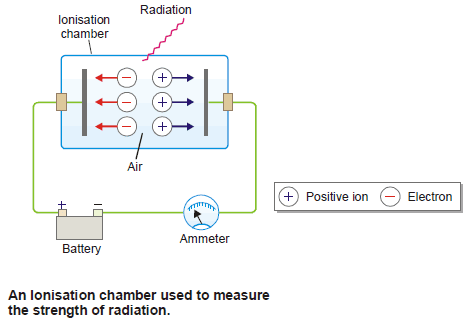
(2) Ionization Chamber
– This is the simplest device used to measure the strength of radiation.
– An ionization chamber is fitted with two metal plates separated by air.
– When radiation passes through this chamber, it knocks electrons from gas molecules, and positive ions are formed.
– The electrons migrate to the anode and positive ions to the cathode.
– Thus a small current passes between the plates.
– This current can be measured with an ammeter and gives the strength of radiation that passes through the ionization chamber.
– In an ionization chamber called a Dosimeter, the total amount of electric charge passing between the plates in a given time is measured.
– This is proportional to the total amount of radiation that has gone through the chamber.
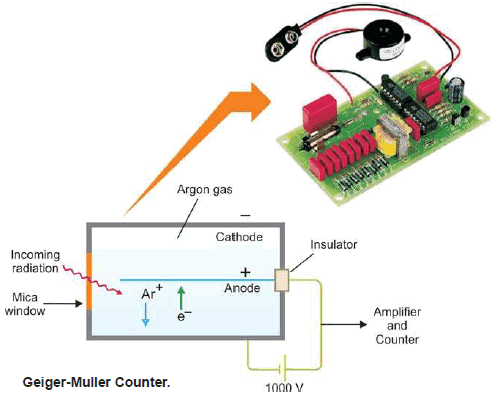
(3) Geiger-Muller Counter
– This device is used for detecting and measuring the rate of emission of α- or β particles.
– It consists of a cylindrical metal tube (cathode) and a central wire (anode).
– The tube is filled with argon gas at reduced pressure (0.1 atm).
– A potential difference of about 1000 volts is applied across the electrodes.
– When an α- or β-particle enters the tube through the mica window, it ionizes the argon atoms along its path.
– The argon ions (Ar+) are drawn to the cathode and electrons to the anode.
– Thus for a fraction of a second, a pulse of electrical current flows between the electrodes and completes the circuit around.
– Each electrical pulse marks the entry of one α- or β-particle into the tube and is recorded in an automatic counter.
– The number of such pulses registered by a radioactive material per minute gives the intensity of its radioactivity.
(4) Scintillation Counter
– Rutherford used a spinthariscope for the detection and counting of α-particles.
– The radioactive substance mounted on the tip of the wire emitted α-particles.
– Each particle on striking the zinc sulfide screen produced a flash of light.
– These flashes of light (scintillations) could be seen through the eye-piece.
– With this device, it was possible to count α-particles from 50 to 200 per second.
Modern scintillation counter
– A modern scintillation counter also works on the above principle and is widely used for the measurement of α- or β-particles.
– Instead of the zinc sulfide screen, a crystal of sodium iodide with a little thallium iodide is employed.
– The sample of the radioactive substance contained in a small vial is placed in a ‘well’ cut into the crystal.
– The radiation from the sample hit the crystal wall and produced scintillations.
– These fall on a photoelectric cell which produces a pulse of electric current for each flash of light. This is recorded on a mechanical counter.
– Such a scintillation counter can measure radiation up to a million per second.
(5) Film Badges
– A film badge consists of a photographic film encased in a plastic holder.
– When exposed to radiation, they darken the grains of silver in photographic film.
– The film is developed and viewed under a powerful microscope.
– As α- or β-particles pass through the film, they leave a track of black particles. These particles can be counted.
– In this way, the type of radiation and its intensity can be known.
– However, γ-radiation darkens the photographic film uniformly.
– The amount of darkening tells the quantity of radiation.
– A film badge is an important device to monitor the extent of exposure of persons working in the vicinity of radiation.
– The badge film is developed periodically to see if any significant dose of radiation has been absorbed by the wearer.
Reference: Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolor edition.




nice explanation and easy to understand