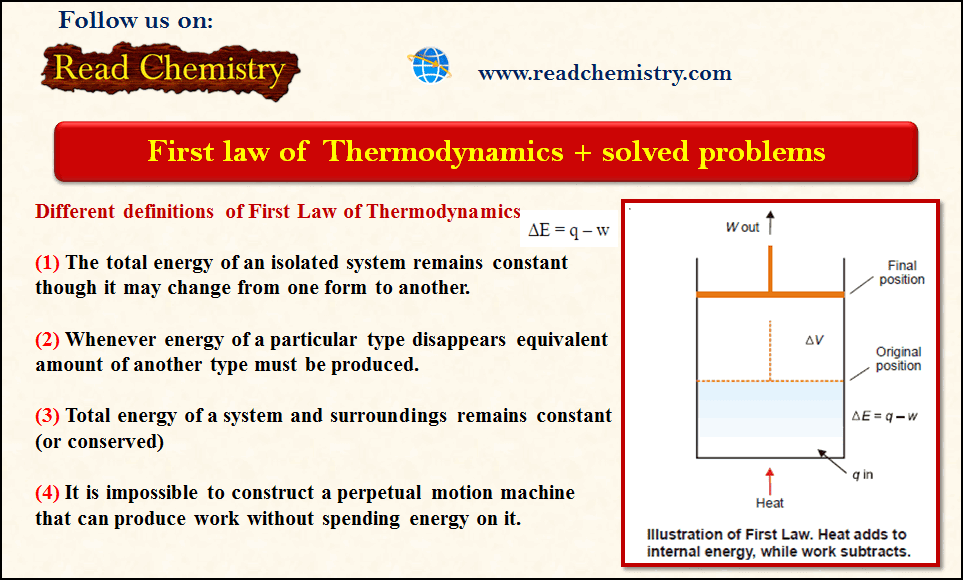
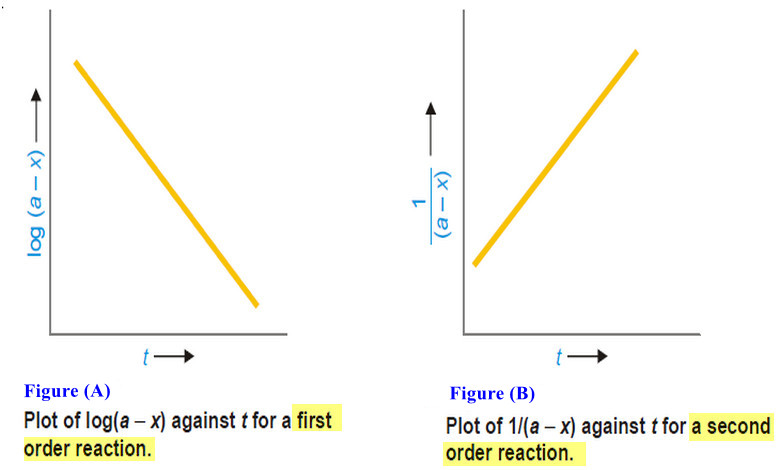
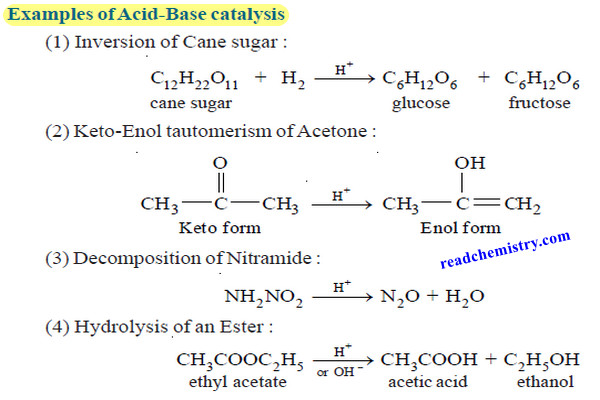
Theories of Osmosis
Theories of Osmosis
– Here we will discuss some theories of osmosis.
– Several theories have been advanced to explain the action of a semipermeable membrane.
– It is probable that the mechanism depends on the particular type of membrane used and also on the nature of the solute and solvent.
– Some of the more important theories of osmosis across a membrane are outlined below:
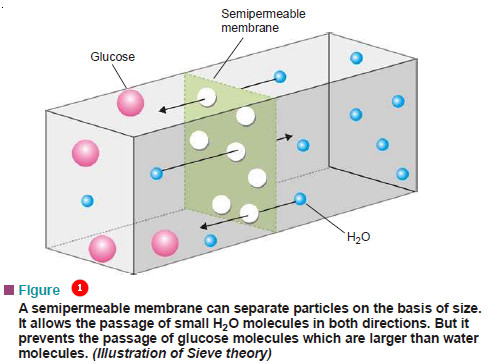
(1) The Molecular Sieve Theory
– According to this theory, the membrane contains lots of fine pores and acts as a sort of ‘molecular sieve’.
– Smaller solvent molecules can pass through the pores but the larger molecules cannot.
– Solvent molecules flow from a region of higher solute concentration to one of lower concentration across such a membrane.
– But since some membranes can act as sieves even though the solute molecules are smaller than the solvent molecules, this theory remains in doubt.
– Recently it has been shown that the pores or capillaries between the protein molecules constituting an animal membrane are lined with polar groups (– COO–, –NH3 +, –S2–, etc.). Therefore, the membrane acts not simply as a sieve but also regulates the passage of solute molecules by electrostatic or ‘chemical interactions’.
– In this way even solute molecules smaller than solvent molecules can be held back by the membrane.
(2) Membrane Solution Theory
– The second theory of theories of osmosis is Membrane Solution Theory.
– Membrane proteins bearing functional groups such as – COOH, – OH, – NH2, etc., dissolve water molecules by hydrogen bonding or chemical interaction.
– Thus membrane dissolves water from the pure water (solvent) forming what may be called ‘membrane solution’.
– The dissolved water flows into the solution across the membrane in a bid to equalise concentrations.
– In this way water molecules pass through the membrane while solute molecules being insoluble in the membrane do not.
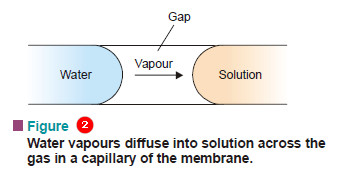
(3) Vapour Pressure Theory
– It suggests that a semipermeable membrane has many fine holes or capillaries. The walls of these capillaries are not wetted by water (solvent) or solution.
– Thus neither solution nor water can enter the capillaries. Therefore each capillary will have in it solution at one end and water at the other, separated by a small gap.
– Since the vapour pressure of a solution is lower than that of the pure solvent, the diffusion of vapour will occur across the gap from water side to solution side.
– This will result in the transfer of water into the solution.
– The vapour theory offers a satisfactory explanation of the mechanism of osmosis in most cases.
(4) Membrane Bombardment Theory
– This theory suggests that osmosis results from an unequal bombardment pressure caused by solvent molecules on the two sides of the semipermeable membrane.
– On one side we have only solvent molecules while on the other side there are solute molecules occupying some of the surface area.
– Thus there are fewer bombardments per unit area of surface on the solution side than on the solvent side.
– Hence the solvent molecules will diffuse more slowly through the membrane on the solution side than on the solvent side.
– The net result causes a flow of the solvent from the pure solvent to the solution across the membrane.
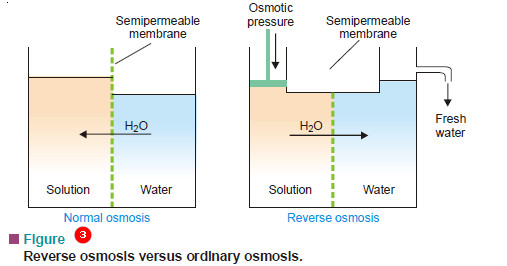
Reverse Osmosis
– When a solution is separated from pure water by a semipermeable membrane, osmosis of water occurs from water to solution. This osmosis can be stopped by applying pressure equal to osmotic pressure, on the solution.
– If pressure greater than osmotic pressure is applied, osmosis is made to proceed in the reverse direction to ordinary osmosis i.e., from solution to water.
– The osmosis taking place from solution to pure water by application of pressure greater than osmotic pressure, on the solution, is termed Reverse Osmosis.
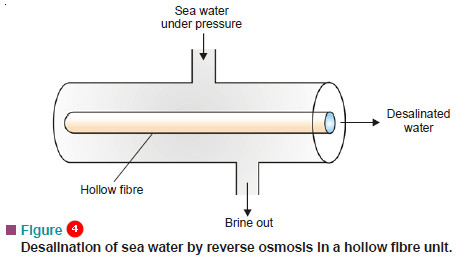
Desalination of Sea Water by Hollow-fibre Reverse Osmosis
– Reverse osmosis is used for the desalination of sea water for getting fresh drinking water.
– This is done with the help of hollow fibres (nylon or cellulose acetate) whose wall acts as semipermeable membrane.
– A hollow-fibre reverse osmosis unit is shown in Fig.4.
– Water is introduced under pressure around the hollow fibres. The fresh water is obtained from the inside of the fibre.
– In actual practice, each unit contains more than three million fibres bundled together, each fibre is of about the diameter of a human hair.