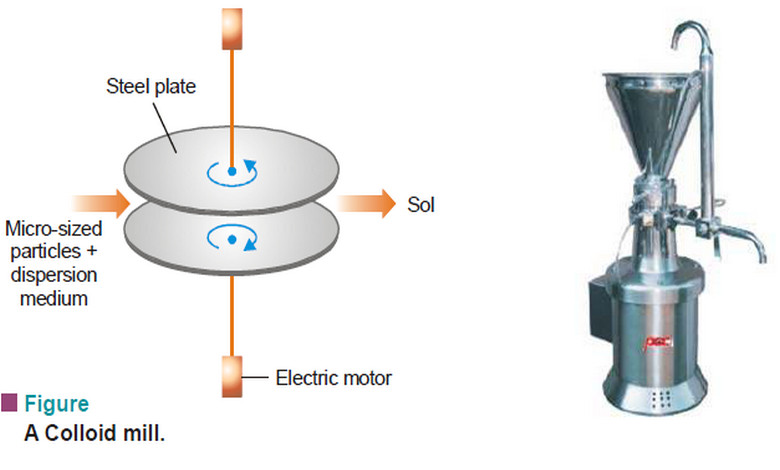
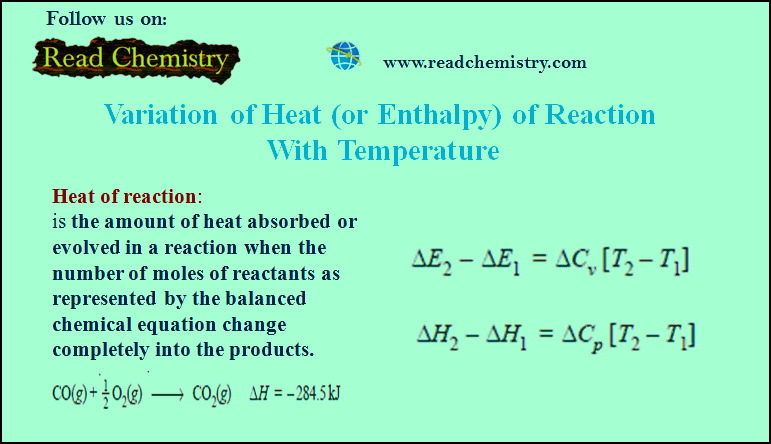
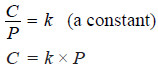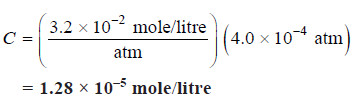Henry’s law – a form of distribution law
Henry’s law statement
– Henry’s law states:
at a constant temperature the solubility of a gas in a liquid is proportional to the pressure of the gas above it.
– Henry’s law may be mathematically expressed as
C = kP
where:
C is the solubility (or concentration),
k is a constant
P is the pressure of the gas,
k is called Henry’s constant.
Explanation of Henry’s law
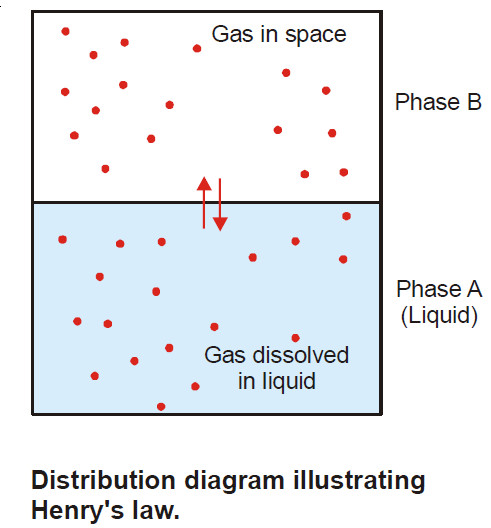
– Henry’s law is, in fact, a form of Distribution law.
– If a vessel containing a liquid and a gas is shaken, at equilibrium the gas can be regarded as distributed between the liquid (Phase A) and the space above (Phase B).
– The influence of partial pressure on solubility is utilized in making carbonated beverages like beer, champagne, and many soft drinks.
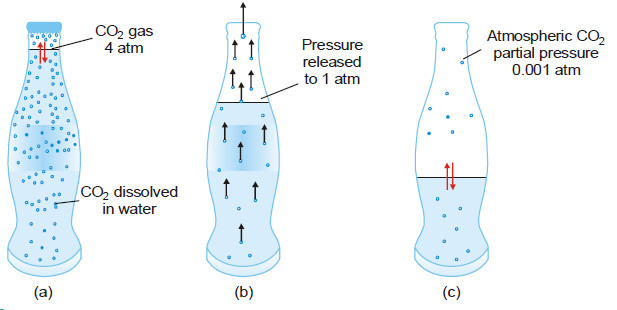
– So called ‘soda water’ is bottled under a carbon dioxide pressure of about 4 atm.
– When the bottle is opened to the air, the partial pressure of CO2 above the solution is decreased (about 0.001 atm), and CO2 bubbles out.
(a) CO gas at 4 atm in equilibrium with dissolved CO2 resulting in high solubility of CO2 ;
(b) In opened bottle pressure is released to 1 atm and hence equilibrium shifted upward, gas bubbles evolved causing brisk effervescence;
(c) Partial pressure of CO2 in air being 0.001 atm, practically the whole of CO2 is removed from solution, leaving the soft drink flat as the equilibrium is established.
Henry’s Law equation
Let:
C1 be the concentration of the gas in phase B
C be the concentration of the gas in phase A
Applying the Distribution law:
We know that molar concentration of gas is proportional to its pressure, P.
This is Henry’s Law equation.
– Like distribution law, Henry’s law holds good for dilute solutions of gases which do not react with the solvent.
– If a mixture of gases is in contact with a liquid, the partial pressure of an individual gas, not their total pressure, determines the mass of each gas dissolving.
– In such a case, the solubility of each gas is proportional to its partial pressure.
Solved problems
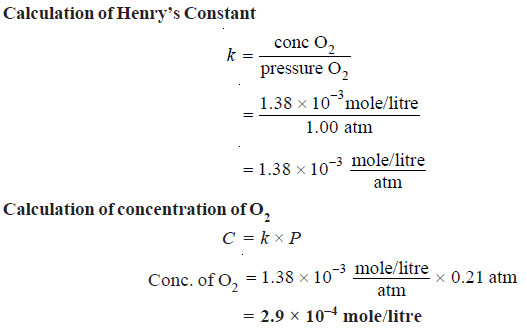
Solved problem(1): The solubility of pure oxygen in water at 20º C and 1.00 atm pressure is 1.38 × 10–3 mole/litre. Calculate the concentration of O2 (mole/litre) at 20ºC and a partial pressure of 0.21 atm.
Solution:
Calculation of Henry’s Constant
Solved problem(1): A soda-water bottle was opened and the soft drink allowed to come to equilibrium at 25ºC with air containing carbon dioxide at a pressure of 4.0 × 10–4 atm. Find the concentration of CO2 in the soda after it had stood open and come to equilibrium. The Henry’s constant for aqueous solution of CO2 at 25º is:
Solution:
C = kP (Henry’s law)
Substituting into Henry’s law equation, we get for the opened soft drink at equilibrium with atmospheric CO2,