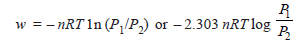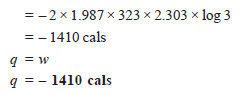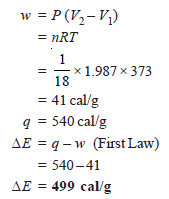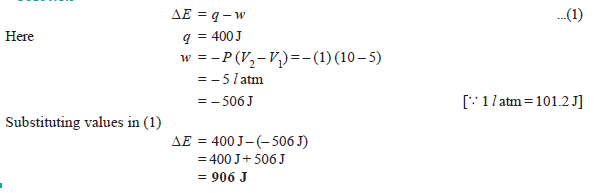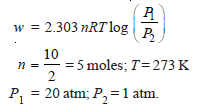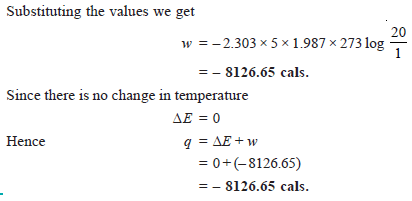The First Law of Thermodynamics + Solved Problems
– The first law of Thermodynamics states that The total energy of an isolated system remains constant though it may change from one form to another.
Internal Energy
– A thermodynamic system containing some quantity of matter has within itself a definite quantity of energy.
– This energy includes not only the translation kinetic energy of the molecules but also other molecular energies such as rotational, and vibrational energies.
– The kinetic and potential energy of the nuclei and electrons within the individual molecules also contribute to the energy of the system.
– Internal Energy is The total of all the possible kinds of energy of a system.
– The word (internal) is often omitted and the energy of a system always implies internal energy.
– The internal energy of a system, like temperature, pressure, volume, etc., is determined by the state of a system and is independent of the path by which it is obtained.
– Hence internal energy of a system is a state function.
– For example, we consider the heating of one mole of liquid water from 0º to 100º C.
– The change in energy is 1.8 kcal and is the same regardless of the form in which this energy is transferred to the water by heating, by performing work, by electrical means, or in any other way.
– Since the value of the internal energy of a system depends on the mass of the matter contained in a system, it is classed as an extensive property.
Symbol Representation of Internal Energy and Sign Conventions
– The internal energy of a system is represented by the symbol (E) (Some books use the symbol U).
– It is neither possible nor necessary to calculate the absolute value of the internal energy of a system.
– In thermodynamics, we are concerned only with the energy changes when a system changes from one state to another.
– If ΔE is the difference in energy of the initial state (Ein) and the final state (Ef), we can write
ΔE = Ef – Ein
ΔE is +ve if Ef is greater than Ein
and ΔE is –ve if Ef is less than Ein.
– A system may transfer energy to or from the surroundings as heat or as work, or both.
Units of Internal Energy
– The SI unit for the internal energy of a system is the joule (J).
– Another unit of energy that is not an SI unit is the calorie, 1 cal = 4.184 J.
The first law of Thermodynamics statement
– The first law of thermodynamics is, in fact, an application of the broad principle known as the Law of Conservation of Energy to the thermodynamic system.
– First law of Thermodynamics states that: The total energy of an isolated system remains constant though it may change from one form to another.
– When a system is changed from state A to state B, it undergoes a change in the internal energy from EA to EB.
– Thus, we can write:
ΔE = EB – EA
– This energy change is brought about by the evolution or absorption of heat and/or by work being done by the system.
– Because the total energy of the system must remain constant, we can write the mathematical statement of the First Law as:
ΔE = q – w …(1)
– where q = the amount of heat supplied to the system, w = work done by the system
– Thus the First Law may also be stated as the net energy change of a closed system is equal to the heat transferred to the system minus the work done by the system.
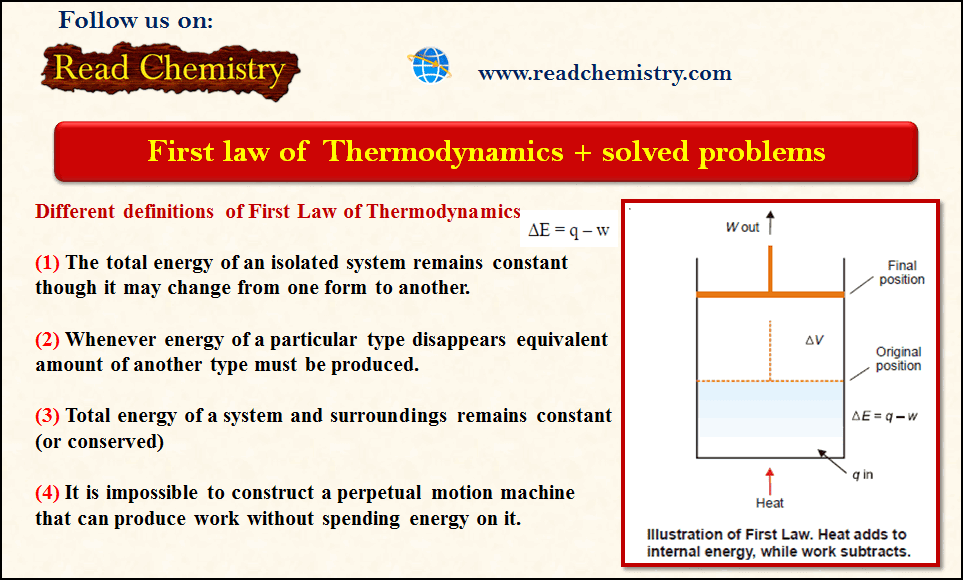
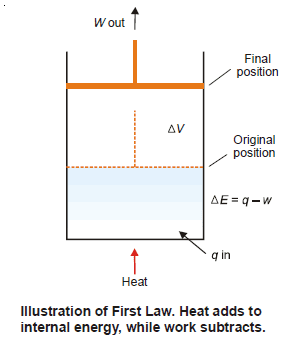
– To illustrate the mathematical statement of the First Law, let us consider the system ‘expanding hot gas’.
– The gas expands against an applied constant pressure by volume ΔV.
– The total mechanical work done is given by the relation:
w = P × ΔV …(2)
– From (1) and (2), we can restate:
ΔE = q – P × ΔV
Other Definitions of the First Law of Thermodynamics
(1) Whenever energy of a particular type disappears equivalent amount of another type must be produced.
(2) Total energy of a system and surroundings remains constant (or conserved)
(3) It is impossible to construct a perpetual motion machine that can produce work without spending energy on it.
Some Special Forms of the First Law of Thermodynamics
– The mathematical statement of the First Law of Thermodynamics is:
ΔE = q – w
Case (1): For a cyclic process involving isothermal expansion of an ideal gas
ΔE = o
∴ q = w
Case (2): For an isochoric process (no change in volume) there is no work of expansion i.e. w = 0.
Hence
ΔE = qv
Case (3): For an adiabatic process there is no change in heat gained or lost i.e. q = 0.
Hence
ΔE = – w
– In other words, the decrease in internal energy is exactly equal to the work done on the system by surroundings.
Case (4): For an isobaric process there is no change in pressure, i.e. P remains constant.
Hence
ΔE = q – w
or ΔE = q – PΔV
Solved Problem
Problem (1): Find ΔE, q, and w if 2 moles of hydrogen at 3 atm pressure expand isothermally at 50ºC and reversibly to a pressure of 1 atm.
Solution:
– Since the operation is isothermal and the gas is ideal
Problem (2): 1g of water at 373 K is converted into steam at the same temperature. The volume of water becomes 1671 ml on boiling. Calculate the change in the internal energy of the system if the heat of vaporization is 540 cal/g.
Solution:
– As the vaporization takes place against a constant pressure of 1 atmosphere, work done for an irreversible process, w, is:
Problem (3): A gas contained in a cylinder fitted with a frictionless piston expands against a constant external pressure of 1 atm from a volume of 5 liters to a volume of 10 liters. In doing so it absorbs 400 J thermal energy from its surroundings. Determine ΔE for the process.
Solution:
Problem (4): Calculate the maximum work done when pressure on 10 g of hydrogen is reduced from 20 to one atmosphere at a constant temperature of 273 K. The gas behaves ideally. Will there be any change in internal energy? Also, calculate ‘q’.
Solution:
Reference: Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolor edition.