What is Osmosis and Osmotic Pressure?
Diffusion and Osmosis
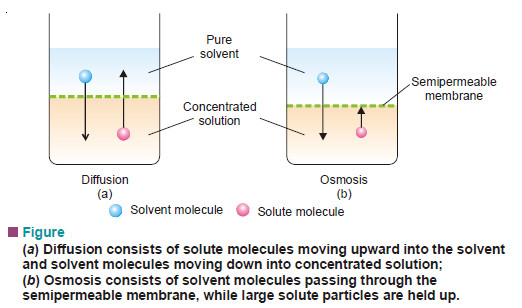
– Just as a gas can diffuse into vacant space or another gas, a solute can diffuse from a solution into the pure solvent.
– If you pour a saturated aqueous solution of potassium permanganate with the help of a thistle funnel into a beaker containing water, it forms a separate layer at the bottom.
– After some time, you will see the permanganate actually diffusing up into water. This continues until a homogeneous solution is obtained.
– The diffusion of solute into solvent is, in fact, a bilateral process. It consists of:
(1) the solute molecules moving up into solvent; and
(2) the solvent molecules moving down into solution.
– This intermingling of solute and solvent molecules goes on, so that ultimately a solution of uniform concentration results.
– It is the tendency to equalise concentration in all parts of the solution which is responsible for the diffusion of the solute.
– Thus diffusion of solute will also take place when two solutions of unequal concentrations are in contact.
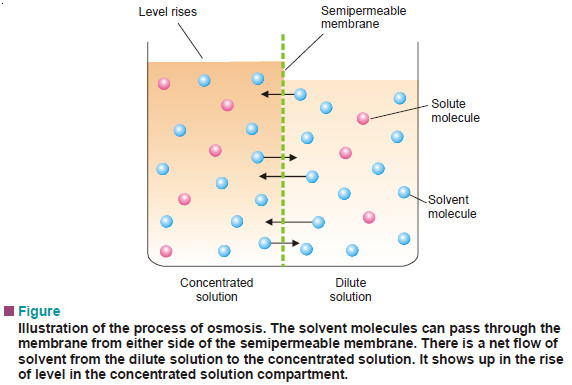
– Solvent molecules will pass from the dilute to the concentrated solution and solute molecules will pass from the concentrated to the dilute solution until equality of concentration is achieved.
What is Osmosis?
– Let us consider a pure solvent and solution separated by a membrane which permits the passage to solvent molecules but not to solute molecules.
– Only the solvent will diffuse through the membrane into solution.
– A membrane which is permeable to solvent and not to solute, is called a semipermeable membrane.
– The flow of the solvent through a semipermeable membrane from pure solvent to solution, or from a dilute solution to concentrated solution, is termed Osmosis (Greek Osmos = to push).
– It must be clearly understood that the diffusion of solvent molecules through a semipermeable membrane is taking place in both directions.
– That is, solvent molecules are passing from solvent to solution, and also from solution to solvent.
– But since the diffusion from solvent to solution or from dilute to concentrated solution, is more rapid, the net flow of the solvent is from low to high concentration.
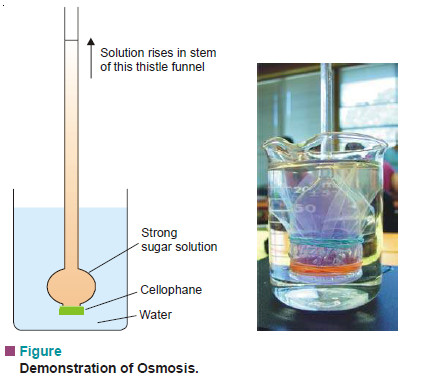
– The phenomenon of osmosis can be demonstrated by fastening a piece of animal bladder or cellophane over a thistle funnel as shown in Fig.above.
– A concentrated aqueous sugar solution is placed inside the thistle funnel which is then immersed in water.
– The osmosis takes place through the semipermeable membrane from water to the sugar solution.
– The flow of water into the funnel shows up as the solution is seen rising in the tube remarkably.
Some interesting Experiments demonstrating Osmosis
– Many natural membranes are semipermeable e.g., pig’s bladder, skin around white of an egg, membrane around the red blood corpuscle, and the membrane in the cell of the plant.
– Some interesting experiments demonstrating osmosis are listed below.
(1) Silica Garden
– Crystals of many salts e.g., ferrous sulphate, nickel chloride, cobalt nitrate and ferric chloride are placed in a solution of glass material (sodium silicate).
– The layers of metallic silicates formed on the surface of crystals by double decomposition are semipermeable.
– The water from outside enters through these membranes which burst and form what we call a Silica Garden.
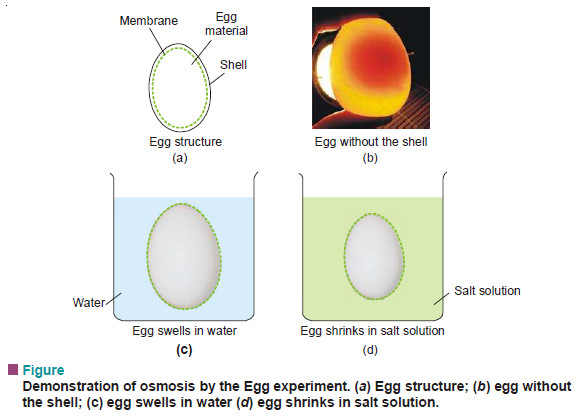
(2) The Egg Experiment
– The outer hard shell of two eggs of the same size is removed by dissolving in dilute hydrochloric acid.
– One of these is placed in distilled water and the other in saturated salt solution.
– After a few hours it will be noticed that the egg placed in water swells and the one in salt solution shrinks.
– In the first case, water diffuses through the skin (a semipermeable membrane) into the egg material which swells.
– In the second case, the concentration of the salt solution being higher than the egg material, the egg shrinks.
Semipermeable Membranes
– Animal and vegetable membranes are not completely semipermeable. Cupric ferrocyanide, Cu2Fe(CN)6, membrane deposited in the walls of a porous pot is perfectly semipermeable and is used for accurate experimental work.
– All semipermeable membranes have fine holes or capillaries in their structure.
– These allow passage to solvent molecules but not to larger solute molecules.
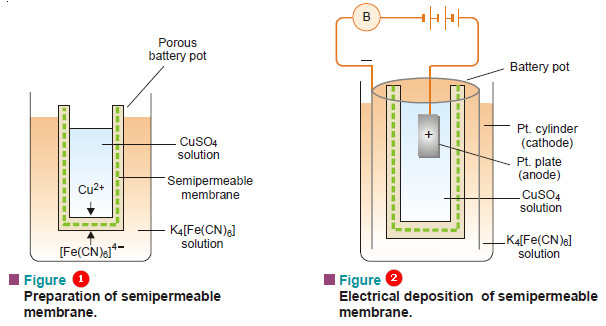
Preparation of Cupric Ferrocyanide Membrane
– An unglazed porcelain battery pot filled with copper sulphate solution is placed in a solution of potassium ferrocyanide, K4Fe(CN)6.
– The two solutions permeate into porous walls of the pot from opposite sides.
– A gelatinous precipitate of cupric ferrocyanide is formed in the middle of the pores of the walls.
– The electrical method which gives a more compact and stout membrane in a short time is shown in Fig.2
– The resistance of the cell rises as the membrane is completely formed and the bell rings no more.
What is Osmotic Pressure?
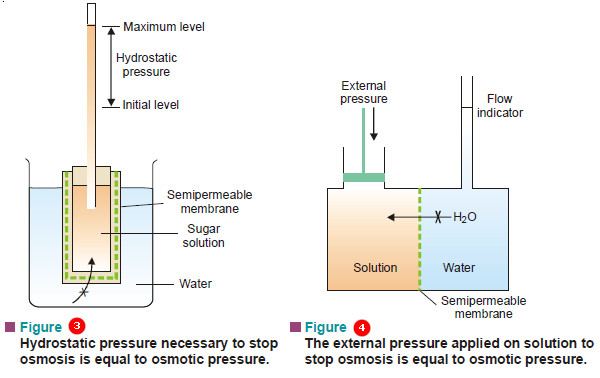
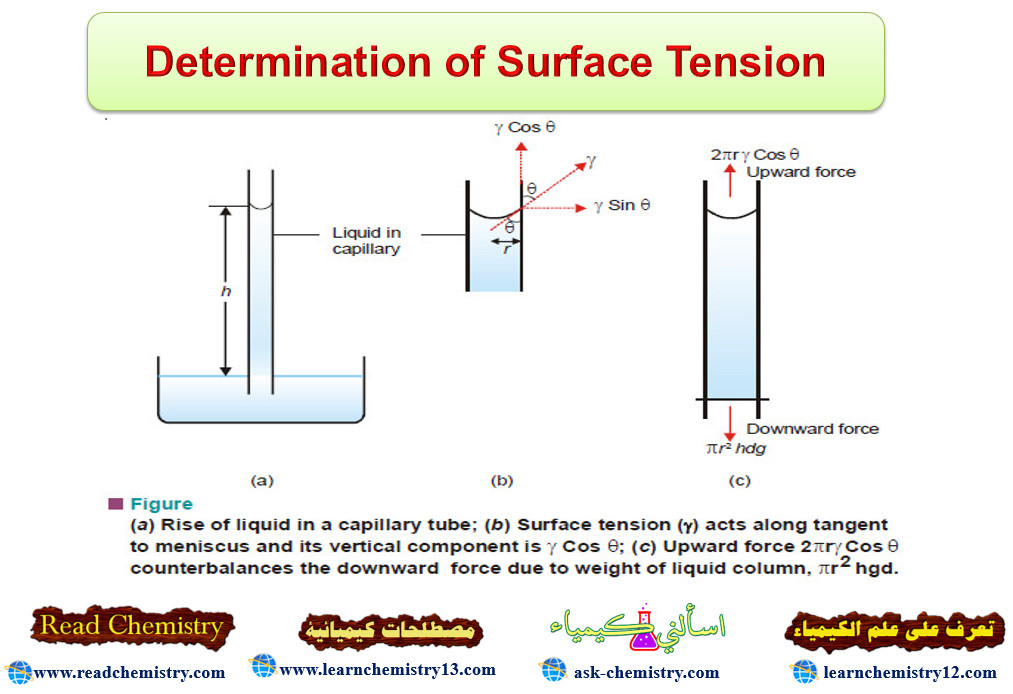
– A porous pot with cupric ferrocyanide membrane deposited in its walls is fitted with a rubber stopper having a long glass tube (Fig. 1).
– It is filled with concentrated aqueous sugar solution and immersed in distilled water.
– The osmosis of water through the membrane from water to the sugar solution takes place.
– As a result, the solution level in the long tube rises over a period of time. After a few days the level attains a definite maximum value.
– This marks the stage when the hydrostatic pressure set up due to the column of sugar solution counterbalances the flow of pure water (or osmosis) into the solution.
– The hydrostatic pressure built up on the solution which just stops the osmosis of pure solvent into the solution through a semipermeable membrane, is called Osmotic Pressure.
– Osmosis can be counteracted not only by hydrostatic pressure but also by application of external pressure on the solution.
– The external pressure may be adjusted so as to prevent the osmosis of pure water into solution. This provides another definition of osmotic pressure.
– Osmotic pressure may be defined as the external pressure applied to the solution in order to stop the osmosis of solvent into solution separated by a semipermeable membrane.
– This definition can be illustrated by means of the apparatus shown in Fig. 2.
– The external pressure on the solution is applied with the help of the piston and the progress of osmosis is shown by the movement of the liquid in the flow indicator.
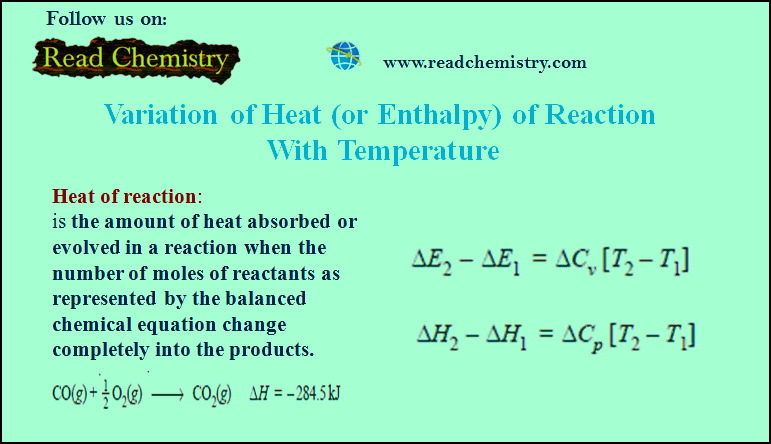
– The pressure required just to arrest the movement of the liquid level in the flow indicator, is equal to the osmotic pressure of the solution.