Autocatalysis, Catalytic poisoning and Negative Catalysis
– In this topic, we will discuss Autocatalysis, Catalytic poisoning and Negative Catalysis.
Catalytic poisoning
– Very often a heterogeneous catalyst in rendered ineffective by the presence of small amounts of impurities in the reactants.
– A substance which destroys the activity of the catalyst to accelerate a reaction, is called a poison and the process is called Catalytic poisoning.
Examples of Catalytic Poisoning
(1) The platinum catalyst used in the oxidation of sulphur dioxide (Contact Process), is poisoned by arsenic oxide (As2O3)
(2) The iron catalyst used in the synthesis of ammonia (Haber Process) is poisoned by H2S.
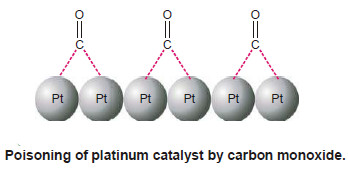
(3) The platinum catalyst used in the oxidation of hydrogen is poisoned by carbon monoxide.
Explanation of Catalytic Poisoning
(1) The poison is adsorbed on the catalyst surface in preference to the reactants.
– Even a monomolecular layer renders the surface unavailable for further adsorption of the reactants.
– The poisoning by As2O3 or CO appears to be of this kind.
(2) The catalyst may combine chemically with the impurity.
– The poisoning of iron catalyst by H2S falls in this class.
Fe + H2S ⎯⎯→ FeS + H2
Autocatalysis
– When one of the products of reaction itself acts as a catalyst for that reaction the phenomenon is called Autocatalysis.
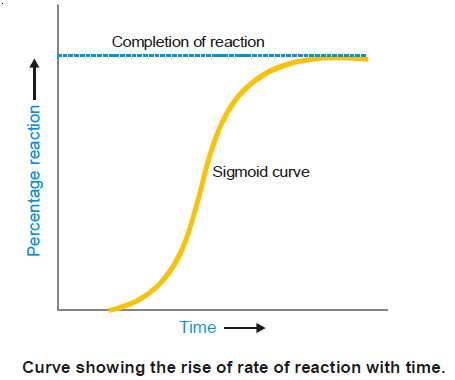
– In autocatalysis the initial rate of the reaction rises as the catalytic product is formed, instead of decreasing steadily.
– The curve plotted between reaction rate and time shows a maximum when the reaction is complete.
Examples of Autocatalysis
(1) Hydrolysis of an Ester.
– The hydrolysis of ethyl acetate forms acetic acid (CH3COOH) and ethanol.
– Of these products, acetic acid acts as a catalyst for the reaction.
CH3COOC2H5 + H2O ⎯⎯→ CH3COOH + C2H5OH
(2) Oxidation of Oxalic acid.
– When oxalic acid is oxidised by acidified potassium permanganate, manganous sulphate produced during the reaction acts as a catalyst for the reaction.
2KMnO4 + 5H2C2O4 + 3H2SO4 ⎯⎯→ 2MnSO4 + K2SO4 + 8H2O + 10CO2
(3) Decomposition of Arsine.
– The free arsenic produced by the decomposition of arsine (AsH3) autocatalyses the reaction.
2AsH3 ⎯⎯→ 2As + 3H2
Negative catalysis
– When a catalyst reduces the rate of a reaction, it is called a Negative catalyst or Inhibitor.
– This phenomenon is called Negative catalysis or Inhibition.
– Negative catalysis is useful to slow down or stop altogether an unwanted reaction.
Examples of Negative Catalysis
(1) Oxidation of Trichloromethane (CHCl3)
– Trichloromethane (or chloroform) is used as anaesthetic.
– Upon oxidation by air it forms carbonyl chloride (COCl2) which is a poisonous substance.
4CHCl3 + 3O2 ⎯⎯→ 4COCl2 + 2H2O + 2Cl2
– 2 per cent of ethanol (C2H5OH) when added to chloroform acts as a negative catalyst and suppresses the formation of carbon chloride.
(2) Decomposition of Hydrogen peroxide
– The decomposition of hydrogen peroxide,
2H2O2 ⎯⎯→ 2H2O + O2
is retarded by the presence of dilute acids or glycerol.
(3) Tetraethyllead as Antiknock
– When tetraethyllead, Pb(C2H5)4, is added to petrol, it retards the too repaid or explosive combustion of the fuel which is responsible for knocking of the engine.
Explanation of Negative Catalysis
– The mechanism of negative catalysis could be different for different reactions.
(1) By poisoning a catalyst.
– A negative catalyst may function by poisoning a catalyst which already happens to be present in the reaction mixture.
– For example, the traces of alkali dissolved from the glass of the container, catalyse the decomposition of hydrogen peroxide (H2O2).
– But the addition of an acid would destroy the alkali catalyst and thus prevent decomposition.
(2) By breaking a chain reaction.
– In some cases negative catalysts are believed to operate by breaking the chain of reactions.
– For example, the combination of H2 and Cl2, which is a chain reaction, is negatively catalysed by nitrogen trichloride (NCl3).
Cl2 ⎯⎯→ Cl. + Cl.
H2 + Cl. ⎯⎯→ HCl + H.
H. + Cl2 ⎯⎯→ HCl + H.
– NCl3 breaks the chain of reactions by absorbing the propagating species (Cl), and the reaction stops.
NCl3 + Cl. → 1/2 N2 + 2Cl2