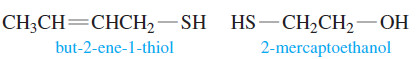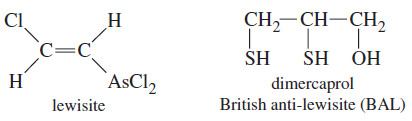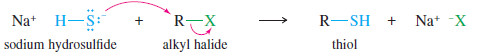Thiols (Mercaptans)
What is Thiols?
– Thiols are sulfur analogues of alcohols, with an -SH group in place of the alcohol -OH group.
– Oxygen and sulfur are in the same column of the periodic table (group 6A), with oxygen in the second row and sulfur in the third.
Nomenclature of Thiols
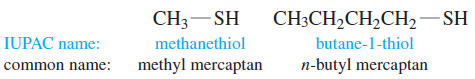
– IUPAC names for their compounds are derived from the alkane names, using the suffix -thiol.
– Thiols are also called mercaptans (captures mercury) because they form stable heavy-metal derivatives.
– Common names are formed like those of alcohols, using the name of the alkyl group with the word mercaptan.
– The -SH group itself is called a mercapto group.
Characteristics of Thiols
– Thiols’ ability to complex heavy metals has proved useful for making antidotes to heavy-metal poisoning.
– For example, in World War II the Allies were concerned that the Germans would use lewisite, a volatile arsenic compound, as a chemical warfare agent.
– Thiols complex strongly with arsenic, and British scientists developed dimercaprol (2,3-dimercaptopropan-1-ol) as an effective antidote.
– The Allies came to refer to this compound as “British anti-lewisite” (BAL), a name that is still used.
– Dimercaprol is useful against a variety of heavy metals, including arsenic, mercury, and gold.
– The odor of thiols is their strongest characteristic.

– Skunk scent is composed mainly of 3-methylbutane-1-thiol and but-2-ene-1-thiol, with small amounts of other thiols.
– Ethanethiol is added to natural gas (odorless methane) to give it the characteristic “gassy” odor for detecting leaks
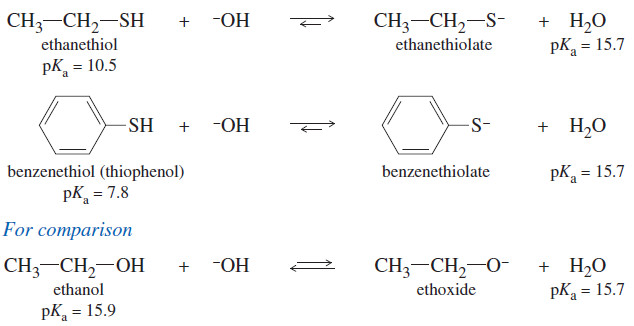
– Although oxygen is more electronegative than sulfur, thiol are more acidic than alcohols.
– Their enhanced acidity results from two effects:
First, S-H bonds are generally weaker than O-H bonds, making S-H bonds easier to break.
Second, the thiolate ion R-S– has its negative charge on sulfur, which allows the charge to be delocalized over a larger region than the negative charge of an alkoxide ion, borne on a smaller oxygen atom.
– Thiolate ions are easily formed by treating the thiol with aqueous sodium hydroxide.
Prepation of Thiols
– Thiols can be prepared by SN2 reactions of sodium hydrosulfide with unhindered alkyl halides.
– The thiol product is still nucleophilic, so a large excess of hydrosulfide is used to prevent the product from undergoing a second alkylation to give a sulfide (R-S-R).
Reactions of Thiols
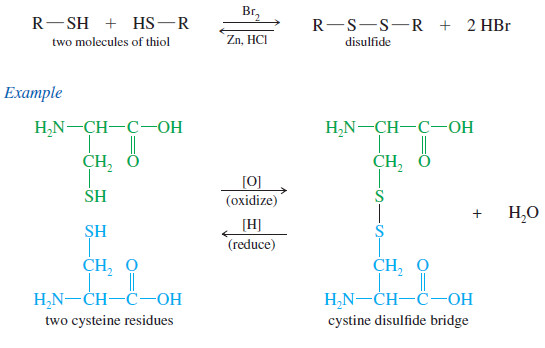
– Unlike alcohols, thiols are easily oxidized to give a dimer called a disulfide.
– The reverse reaction, reduction of the disulfide to the thiol, takes place under reducing conditions.
– Formation and cleavage of disulfide linkages is an important aspect of protein chemistry , where disulfide “bridges” between cysteine amino acid residues hold the protein chain in its active conformation.
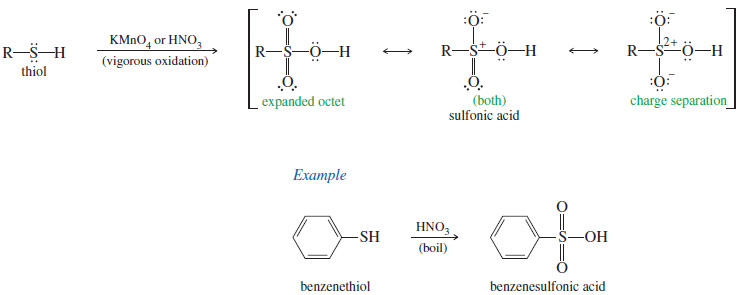
– Just as mild oxidation converts thiols to disulfides, vigorous oxidation converts them to sulfonic acids.
– KMnO4 or nitric acid HNO3 or even bleach (NaOCl), can be used as the oxidant for this reaction.
– Any Lewis structure of a sulfonic acid requires either separation of formal charges or more than 8 electrons around sulfur.
– Sulfur can have an expanded octet, as it does in SF4 (10 electrons) and SF6 (12 electrons).
– The three resonance forms shown here are most commonly used.
– Organic chemists tend to use the form with an expanded octet, and inorganic chemists tend to use the forms with charge separation.