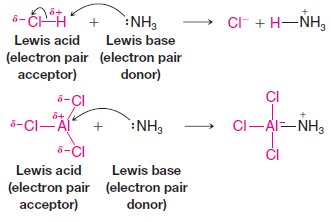
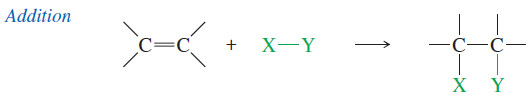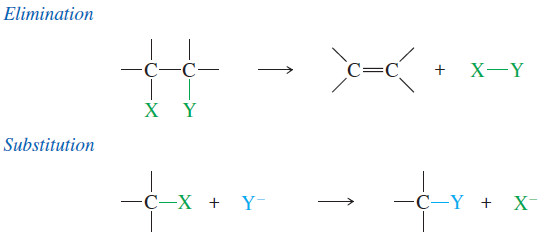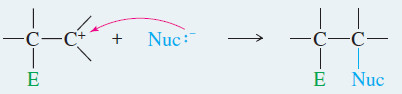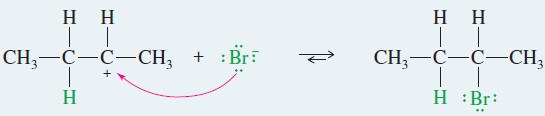Electrophilic Addition to Alkenes
Electrophilic Addition to Alkenes
Reactivity of the Carbon–Carbon Double Bond
– All alkenes have a common feature: a carbon–carbon double bond.
– The reactions of alkenes arise from the reactivity of the carbon–carbon double bond.
– Once again, the concept of the functional group helps to organize and simplify the study of chemical reactions.
– By studying the characteristic reactions of the double bond, we can predict the reactions of alkenes we have never seen before.
– Because single bonds (sigma bonds) are more stable than pi bonds, the most common reactions of double bonds transform the pi bond into a sigma bond.
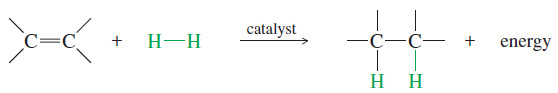
– For example, catalytic hydrogenation converts the (C=C) pi bond and the (H-H) sigma bond into two (C-H) sigma bonds.
– The reaction is exothermic (ΔHo = about -80 to -120 kj/mol or about -20 to -30 kcal/mol), showing that the product is more stable than the reactants.
– Hydrogenation of an alkene is an example of an addition, one of the three major reaction types we have studied: addition, elimination, and substitution.
– In an addition, two molecules combine to form one product molecule.
– When an alkene undergoes addition, two groups add to the carbon atoms of the double bond and the carbons become saturated.
– In many ways, addition is the reverse of elimination, in which one molecule splits into two fragment molecules.
– In a substitution, one fragment replaces another fragment in a molecule.
– Addition is the most common reaction of alkenes, and in this chapter we consider additions to alkenes in detail.
– A wide variety of functional groups can be formed by adding suitable reagents to the double bonds of alkenes
Electrophilic Addition to Alkenes
– In principle, many different reagents could add to a double bond to form more stable products; that is, the reactions are energetically favorable.
– Not all of these reactions have convenient rates, however. For example, the reaction of ethylene with hydrogen (to give ethane) is strongly exothermic, but the rate is very slow.
– A mixture of ethylene and hydrogen can remain for years without appreciable reaction.
– Adding a catalyst such as platinum, palladium, or nickel allows the reaction to take place at a rapid rate.
– Some reagents react with carbon–carbon double bonds without the aid of a catalyst.
What types of reagents react with double bonds?
– To understand what types of reagents react with double bonds, consider the structure of the pi bond. Although the electrons in the sigma bond framework are tightly held, the pi bond is delocalized above and below the sigma bond (see Figure).
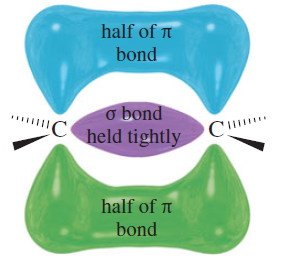
– The pi-bonding electrons are spread farther from the carbon nuclei, and they are more loosely held.
– A strong electrophile has an affinity for these loosely held electrons.
– It can pull them away to form a new bond (see Figure), leaving one of the carbon atoms with only three bonds and a positive charge: a carbocation.
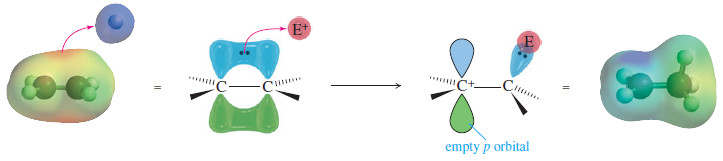
– In effect, the double bond has reacted as a nucleophile, donating a pair of electrons to the electrophile.
– Most addition reactions involve a second step in which a nucleophile attacks the carbocation (as in he second step of the SN1 reaction), forming a stable addition product.
– In the product, both the electrophile and the nucleophile are bonded to the carbon atoms that were connected by the double bond.
– This reaction is outlined in Key Mechanism showen down , identifying the electrophile as E+and the nucleophile as Nuc:–
– This type of reaction requires a strong electrophile to attract the electrons of the pi bond and generate a carbocation in the rate-limiting step.
– Most alkene reactions fall into this large class of electrophilic additions to alkenes.
Mechanism: Electrophilic Addition to Alkenes
– A wide variety of electrophilic additions to Alkenes involve similar mechanisms.
– First, a strong electrophile attracts the loosely held electrons from the pi bond of an alkene.
– The electrophile forms a sigma bond to one of the carbons of the (former) double bond, while the other carbon becomes a carbocation.
– The carbocation (a strong electrophile) reacts with a nucleophile (often a weak nucleophile) to form another sigma bond.
Step 1: Attack of the pi bond on the electrophile forms a carbocation.
Step 2: Attack by a nucleophile gives the addition product.
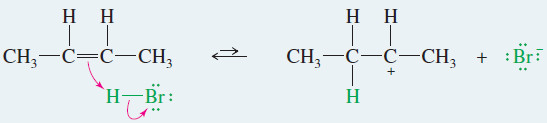
EXAMPLE: Ionic addition of HBr to but-2-ene
– This example shows what happens when gaseous HBr adds to but-2-ene.
– The proton in HBr is electrophilic; it reacts with the alkene to form a carbocation.
– Bromide ion reacts rapidly with the carbocation to give a stable product in which the elements of HBr have added to the ends of the double bond.
Step 1: Protonation of the double bond forms a carbocation.
Step 2: Bromide ion attacks the carbocation.
– We will consider several types of additions to alkenes, using a wide variety of reagents: water, borane, hydrogen, carbenes, halogens, oxidizing agents, and even other alkenes. Most, but not all, of these will be electrophilic additions.
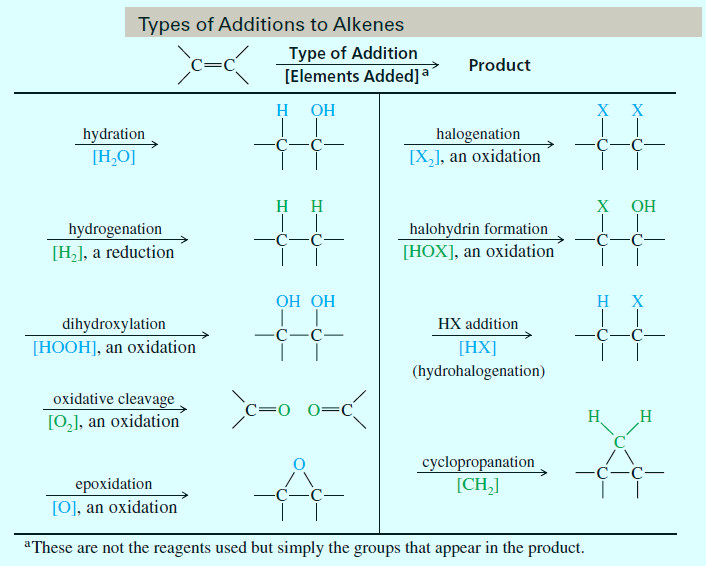
Types of Additions to Alkenes
– The following table summarizes the classes of additions we will cover.
– Note that the table shows what elements have added across the double bond in the final product, but it says nothing about reagents or mechanisms.
– As we study these reactions, you should note the regiochemistry of each reaction, also called the orientation of addition, meaning which part of the reagent adds to which end of the double bond.
– Also note the stereochemistry if the reaction is stereospecific