Conformations of Cyclohexane: The Chair and the Boat
– In this subject, we will discuss Conformations of Cyclohexane: The Chair and the Boat
Conformations of Cyclohexane: The Chair and the Boat
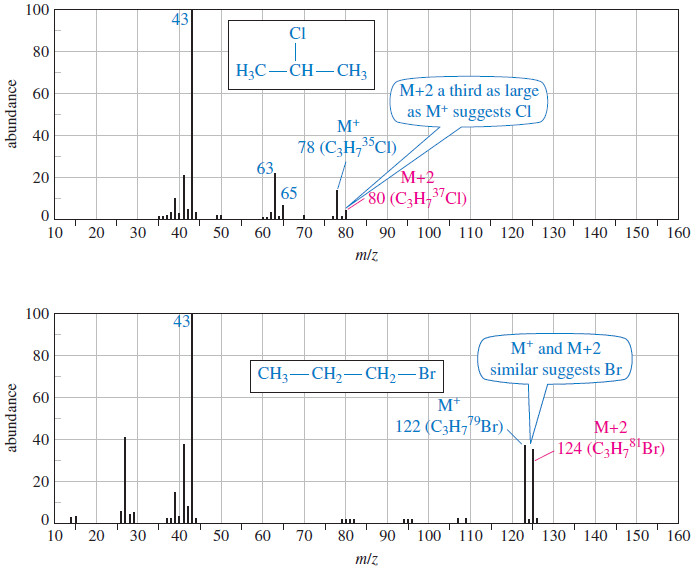
Cyclohexane is more stable than the other cycloalkanes we have discussed, and it has several conformations that are important for us to consider.
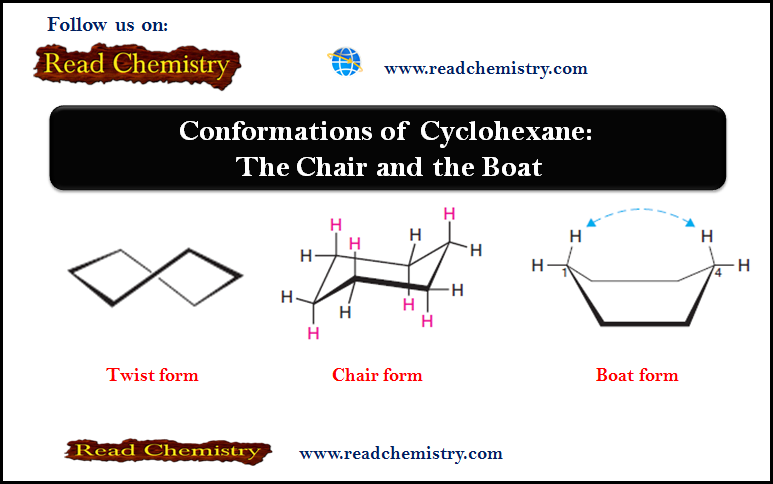
(1) The most stable conformation of cyclohexane is the chair conformation.
(2) There is no angle or torsional strain in the chair form of cyclohexane.
– In a chair conformation (Fig.1), all of the carbon–carbon bond angles are 109.5o and are thereby free of angle strain.
– The chair conformation is free of torsional strain, as well.
– When viewed along any carbon–carbon bond (viewing the structure from an end, Fig.2), the bonds are seen to be perfectly staggered.
– Moreover, the hydrogen atoms at opposite corners of the cyclohexane ring are maximally separated.
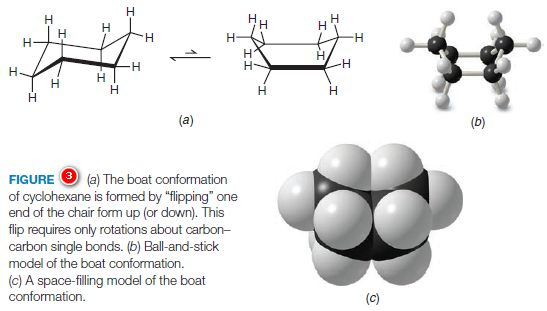
(3) By partial rotations about the carbon–carbon single bonds of the ring, the chair conformation can assume another shape called the boat conformation (Fig.3).
(4) The boat conformation has no angle strain, but it does have torsional strain.
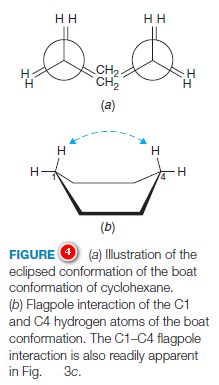
When a model of the boat conformation is viewed down carbon–carbon bond axes along either side (Fig.4a), the C-H bonds at those carbon atoms are found to be eclipsed, causing torsional strain.
Additionally, two of the hydrogen atoms on C1 and C4 are close enough to each other to cause van der Waals repulsion (Fig.4b). This latter effect has been called the “flagpole” interaction of the boat conformation.
Torsional strain and flagpole interactions cause the boat conformation to have considerably higher energy than the chair conformation.
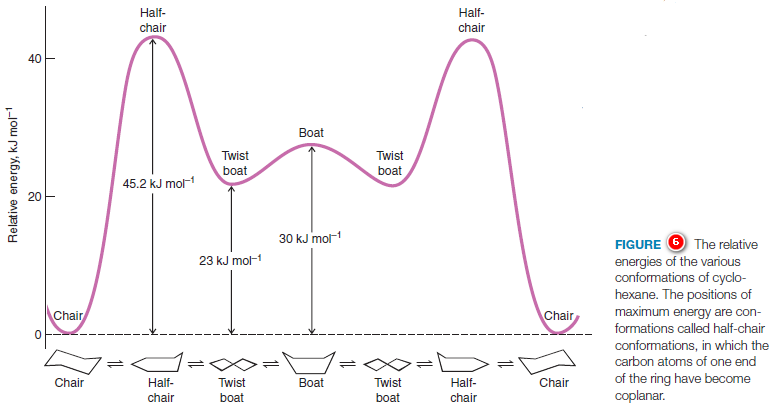
Although it is more stable, the chair conformation is much more rigid than the boat conformation. The boat conformation is quite flexible. By flexing to a new form—the twist conformation (Fig.5)—the boat conformation can relieve some of its torsional strain and, at the same time, reduce the flagpole interactions.
(5) The twist boat conformation of cyclohexane has a lower energy than the pure boat conformation, but is not as stable as the chair conformation.
The stability gained by flexing is insufficient, however, to cause the twist conformation to be more stable than the chair conformation.
The chair conformation is estimated to be lower in energy than the twist conformation by approximately 23 kJ mol-1.
The energy barriers between the chair, boat, and twist conformations of cyclohexane are low enough (Fig.6) to make separation of the conformers impossible at room temperature. At room temperature the thermal energies of the molecules are great enough to cause approximately 1 million interconversions to occur each second.
(6) Because of the greater stability of the chair, more than 99% of the molecules are estimated to be in a chair conformation at any given moment.
Conformations of Higher Cycloalkanes
– Cycloheptane, cyclooctane, cyclononane, and other higher cycloalkanes also exist in nonplanar conformations.
– The small instabilities of these higher cycloalkanes appear to be caused primarily by torsional strain and repulsive dispersion forces between hydrogen atoms across rings, called transannular strain.
– The nonplanar conformations of these rings, however, are essentially free of angle strain.
– X-ray crystallographic studies of cyclodecane reveal that the most stable conformation has carbon–carbon–carbon bond angles of 117o.
– This indicates some angle strain.
– The wide bond angles apparently allow the molecules to expand and thereby minimize unfavorable repulsions between hydrogen atoms across the ring.
– There is very little free space in the center of a cycloalkane unless the ring is quite large.
– Calculations indicate that cyclooctadecane, for example, is the smallest ring through which a -CH2CH2CH2– chain can be threaded.
– Molecules have been synthesized, however, that have large rings threaded on chains and that have large rings that are interlocked like links in a chain.
– These latter molecules are called catenanes.
– In 1994 J. F. Stoddart and co-workers, then at the University of Birmingham (England), achieved a remarkable synthesis of a catenane containing a linear array of five interlocked rings.
– Because the rings are interlocked in the same way as those of the Olympic symbol, they named the compound Olympiadane.
Reference: Organic chemistry / T.W. Graham Solomons, Craig B.Fryhle, Scott A. Snyder, / ( eleventh edition) / 2014.