Drawing Fischer Projections
In this subject we will discuss How to draw Fischer projections
Introduction to Fischer Projections
– We have been using dashed lines and wedges to indicate perspective in drawing the stereochemistry of asymmetric carbon atoms.
– When we draw molecules with several asymmetric carbons, perspective drawings become time consuming and cumbersome.
– In addition, the complicated drawings make it difficult to see the similarities and differences in groups of stereoisomers.
– At the turn of the twentieth century, Emil Fischer was studying the stereochemistry of sugars , which contain as many as seven asymmetric carbon atoms.
– To draw these structures in perspective would have been difficult, and to pick out minor stereochemical differences in the drawings would have been nearly impossible.
– Fischer developed a symbolic way of drawing asymmetric carbon atoms, allowing them to be drawn rapidly.
– The Fischer projection also facilitates comparison of stereoisomers, holding them in their most symmetric conformation and emphasizing any differences in stereochemistry.
Drawing Fischer Projections
– The Fischer projection looks like a cross, with the asymmetric carbon (usually not drawn in) at the point where the lines cross.
– The horizontal lines are taken to be wedges—that is, bonds that project out toward the viewer.
– The vertical lines are taken to project away from the viewer, as dashed lines.
– The following figure shows the perspective implied by the Fischer projection.
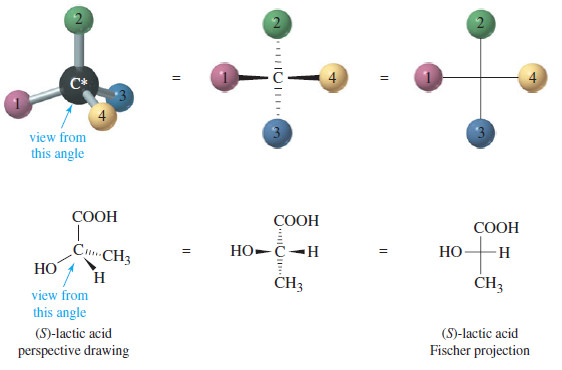
– The center drawing, with the wedged horizontal bonds looking like a bow tie, illustrates why this projection is sometimes called the “bow-tie convention.”
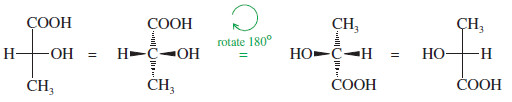
Rotation of a Fischer projection by 180°
– When we rotate a Fischer projection by 180°, the vertical (dashed line) bonds still end up vertical, and the horizontal (wedged) lines still end up horizontal.
– The “horizontal lines forward, vertical lines back” convention is maintained.
Rotation by 180° is allowed.
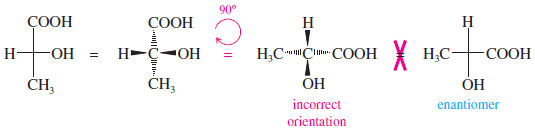
Rotation of a Fischer projection by 90°
– On the other hand, if we were to rotate a Fischer projection by 90°, we would change the configuration and confuse the viewer.
– The original projection has the vertical groups back (dashed lines) and the horizontal groups forward.
– When we rotate the projection by 90°, the vertical bonds become horizontal and the horizontal bonds become vertical.
– The viewer assumes that the horizontal bonds come forward and that the vertical bonds go back.
– The viewer sees a different molecule (actually, the enantiomer of the original molecule).
A 90° rotation is NOT allowed
– In comparing Fischer projections, we cannot rotate them by 90°, and we cannot flip them over.
– Either of these operations gives an incorrect representation of the molecule.
– The Fischer projection must be kept in the plane of the paper, and it may be rotated only by 180°.
– The final rule for drawing Fischer projections helps to ensure that we do not rotate the drawing by 90°.
– This rule is that the carbon chain is drawn along the vertical line of the Fischer projection, usually with the IUPAC numbering from top to bottom.
– In most cases, this numbering places the most highly oxidized carbon substituent at the top.
– For example, to represent (R)-propane-1,2-diol with a Fischer projection, we should arrange the three carbon atoms along the vertical. C1 is placed at the top, and C3 at the bottom.
Drawing Mirror Images of Fischer Projections
– How does one draw the mirror image of a molecule drawn in Fischer projection? With our perspective drawings, the rule was to reverse left and right while keeping the other directions (up and down, front and back) in their same positions. This rule still applies to Fischer projections.
– Interchanging the groups on the horizontal part of the cross reverses left and right while leaving the other directions unchanged.
– Testing for enantiomerism is particularly simple using Fischer projections.
– If the Fischer projections are properly drawn (carbon chain along the vertical), and if the mirror image cannot be made to look the same as the original structure with a 180° rotation in the plane of the paper, the two mirror images are enantiomers.
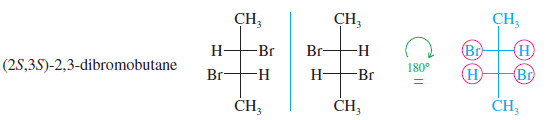
– In the following examples, any groups that fail to superimpose after a 180° rotation are circled in red.
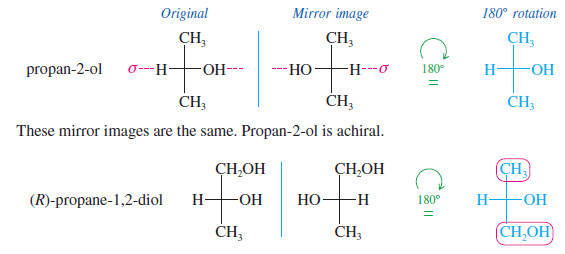
These mirror images are different. Propane-1,2-diol is chiral.
These mirror images are different. This structure is chiral.
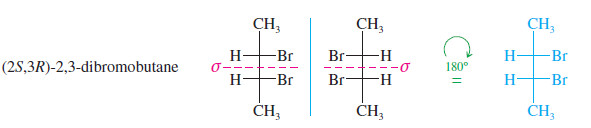
– Mirror planes of symmetry are particularly easy to identify from the Fischer projection because this projection is normally the most symmetric conformation.
– In the first preceding example (propan-2-ol) and in the following example [(2S,3R)-2,3-dibromobutane], the symmetry planes are indicated in red; these molecules with symmetry planes cannot be chiral.
These mirror images are the same. This structure is achiral.
Assigning (R) and (S) Configurations from Fischer Projections
The Cahn–Ingold–Prelog convention can be applied to structures drawn using Fischer projections.
– Let’s review the two rules for assigning (R) and (S):
(1) Assign priorities to the groups bonded to the asymmetric carbon atom;
(2) put the lowest priority group (usually H) in back, and draw an arrow from group 1 to group 2 to group 3.
Clockwise is (R), and counterclockwise is (S).
– The (R) or (S) configuration can also be determined directly from the Fischer projection, without having to convert it to a perspective drawing.
– The lowest-priority atom is usually hydrogen. In the Fischer projection, the carbon chain is along the vertical line, so the hydrogen atom is usually on the horizontal line and projects out in front.
– Once we have assigned priorities, we can draw an arrow from group 1 to group 2 to group 3 and see which way it goes.
– If the molecule were turned around so that the hydrogen would be in back [as in the definition of (R) and (S)], the arrow would rotate in the other direction.
– By mentally turning the arrow around (or simply applying the rule backward), we can assign the configuration.
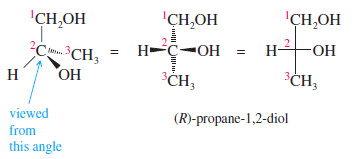
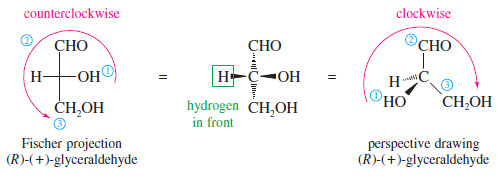
– As an example, consider the Fischer projection formula of one of the enantiomers of glyceraldehyde.
– First priority goes to the ¬OH group, followed by the ¬CHO group and the ¬CH2OH group. The hydrogen atom receives the lowest priority.
– The arrow from group 1 to group 2 to group 3 appears counterclockwise in the Fischer projection.
– If the molecule is turned over so the hydrogen is in back, the arrow is clockwise, so this is the (R) enantiomer of glyceraldehyde.
SUMMARY: Fischer Projections and Their Use
(1) They are most useful for compounds with two or more asymmetric carbon atoms.
(2) Asymmetric carbons are at the centers of crosses.
(3) The vertical lines project away from the viewer, the horizontal lines toward the viewer (like a bow tie ).
(4) The carbon chain is placed along the vertical, with the IUPAC numbering from top to bottom. In most cases, this places the more oxidized end. (the carbon with the most bonds to O or halogen) at the top.
(5) The entire projection can be rotated 180° (but not 90°) in the plane of the paper without changing its stereochemistry.
(6) Interchanging any two groups on an asymmetric carbon (for example, those on the horizontal line) inverts its stereochemistry.