Cyclohexane: Axial and Equatorial Bonds in Cyclohexane
– In this subject, we will discuss the Substituted Cyclohexane: Axial and Equatorial Hydrogen Groups
Substituted Cyclohexane: Axial and Equatorial Hydrogen Groups
– The six-membered ring is the most common ring found among nature’s organic molecules.
– For this reason, we shall give it special attention.
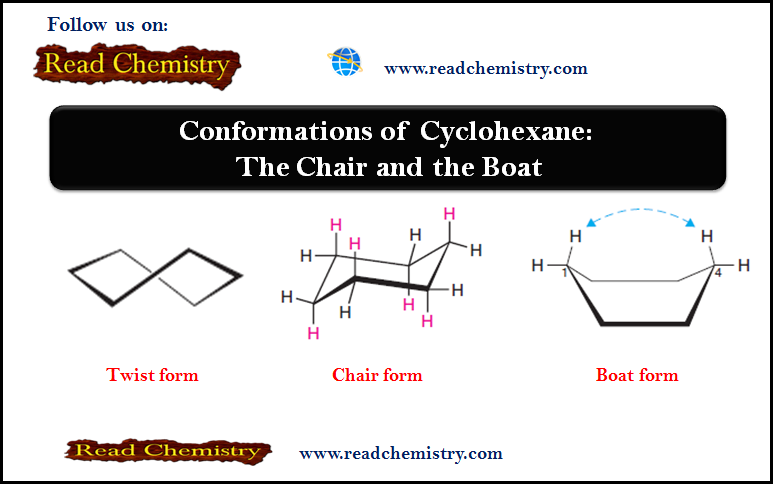
– We have already seen that the chair conformation of cyclohexane is the most stable one and that it is the predominant conformation of the molecules in a sample of cyclohexane.
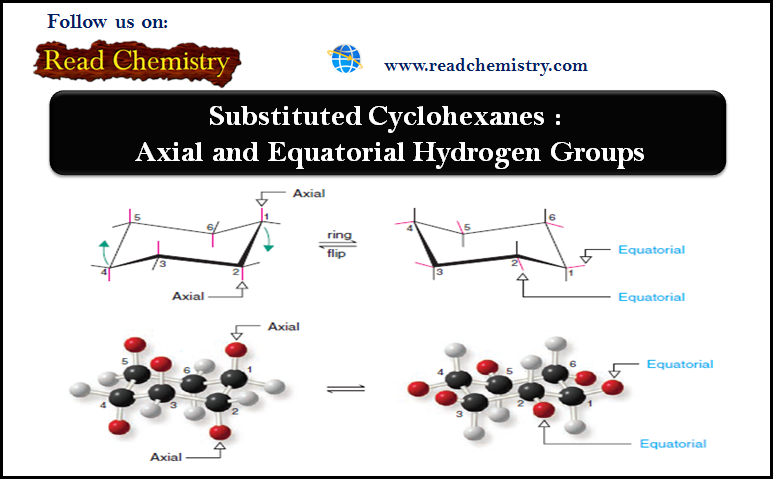
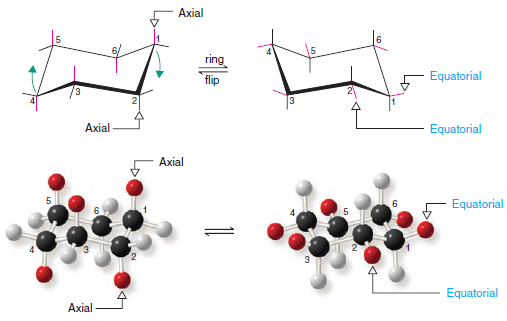
– The chair conformation of a cyclohexane ring has two distinct orientations for the bonds that project from the ring.
– These positions are called axial and equatorial, as shown for cyclohexane in Fig.1.

– The axial bonds of cyclohexane are those that are perpendicular to the average plane of the ring.
– There are three axial bonds on each face of the cyclohexane ring, and their orientation (up or down) alternates from one carbon to the next.
– The equatorial bonds of cyclohexane are those that extend from the perimeter of the ring.
– The equatorial bonds alternate from slightly up to slightly down in their orientation from one carbon to the next.
– When a cyclohexane ring undergoes a chair–chair conformational change (a ring flip), all of the bonds that were axial become equatorial, and all bonds that were equatorial become axial.
How To Draw Chair Conformational Structures
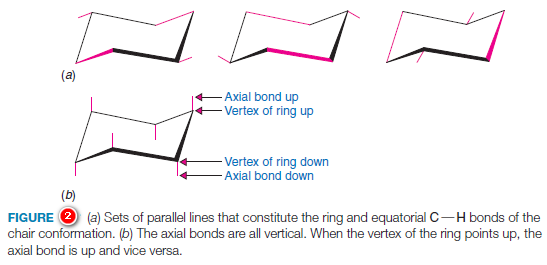
– A set of guidelines will help you draw chair conformational structures that are clear and that have unambiguous axial and equatorial bonds.
(1) Notice in Fig.2a that sets of parallel lines define opposite sides of the chair.
(2) Notice, too, that equatorial bonds are parallel to ring bonds that are one bond away from them in either direction.
(3) When you draw chair conformational structures, try to make the corresponding bonds parallel in your drawings.
(4) When a chair formula is drawn as shown in Fig.8, the axial bonds are all either up or down, in a vertical orientation (Fig.2b).
(5) When a vertex of bonds in the ring points up, the axial bond at that position is also up, and the equatorial bond at the same carbon is angled slightly down.
– When a vertex of ring bonds is down, the axial bond at that position is also down, and the equatorial bond is angled slightly upward.
– Now, try to draw some chair conformational structures for yourself that include the axial and equatorial bonds. Then, compare your drawings with those here and with actual models.
– You will see that with a little practice, your chair conformational structures can be perfect.
A Conformational Analysis of Methylcyclohexane
– Now let us consider methylcyclohexane.
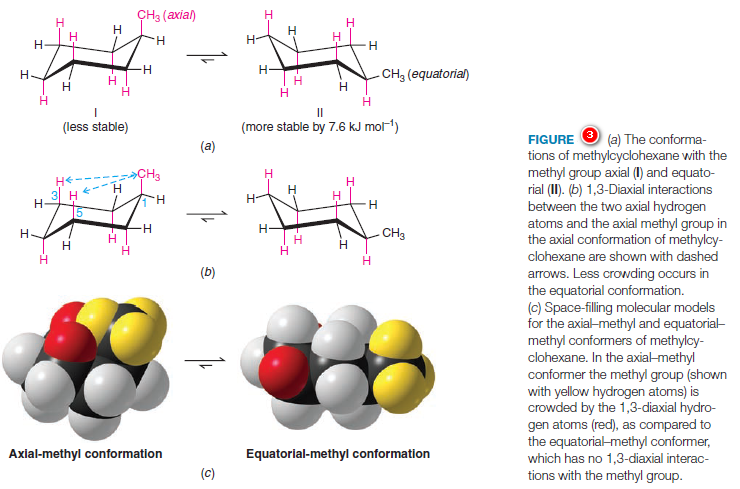
– Methylcyclohexane has two possible chair conformations (Fig.3, I, and II), and these are interconvertible through the bond rotations that constitute a ring flip.
– In conformation (Fig.3a) the methyl group (with yellow hydrogens in the space-filling model) occupies an axial position, and in conformation II the methyl group occupies an equatorial position.
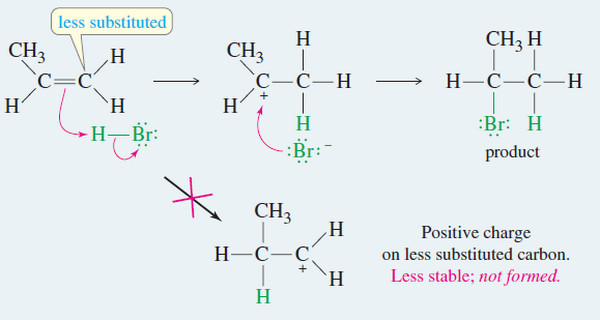
– The most stable conformation for a monosubstituted cyclohexane ring (a cyclohexane ring where one carbon atom bears a group other than hydrogen) is the conformation where the substituent is equatorial.
– Studies indicate that conformation II with the equatorial methyl group is more stable than conformation I with the axial methyl group by about 7.6 kJ mol-1.
– Thus, in the equilibrium mixture, the conformation with the methyl group in the equatorial position is the predominant one, constituting about 95% of the equilibrium mixture.
– The greater stability of methylcyclohexane with an equatorial methyl group can be understood through an inspection of the two forms as they are shown in Figs. 3a–c.
– Studies done with models of the two conformations show that when the methyl group is axial, it is so close to the two axial hydrogens on the same side of the ring (attached to the C3 and C5 atoms) that the dispersion forces between them are repulsive.
– This type of steric strain, because it arises from an interaction between an axial group on carbon atom 1 and an axial hydrogen on carbon atom 3 (or 5), is called a 1,3-diaxial interaction.
– Studies with other substituents show that there is generally less repulsion when any group larger than hydrogen is equatorial rather than axial.
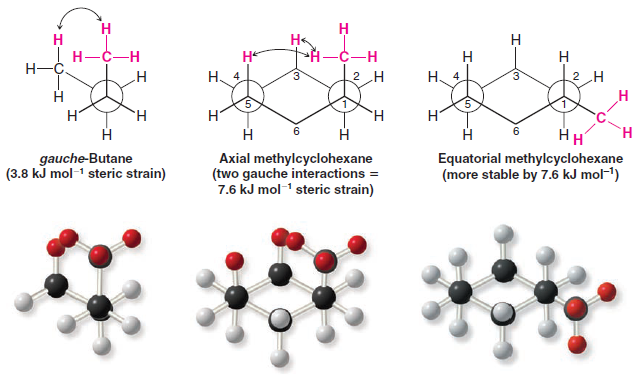
– The strain caused by a 1,3-diaxial interaction in methylcyclohexane is the same as the strain caused by the close proximity of the hydrogen atoms of methyl groups in the gauche form of butane.
– Recall that the interaction in gauche-butane (called, for convenience, a gauche interaction) causes gauche-butane to be less stable than antibutane by 3.8 kJ mol-1.
– The following Newman projections will help you to see that the two steric interactions are the same.
– In the second projection, we view axial methylcyclohexane along the C1-C2 bond and see that what we call a 1,3-diaxial interaction is simply a gauche interaction between the hydrogen atoms of the methyl group and the hydrogen atom at C3
– Viewing methylcyclohexane along the C1-C6 bond (do this with a model) shows that it has a second identical gauche interaction between the hydrogen atoms of the methyl group and the hydrogen atom at C5.
– The methyl group of axial methylcyclohexane, therefore, has two gauche interactions and, consequently, it has 7.6 kJ mol-1 of strain.
– The methyl group of equatorial methylcyclohexane does not have a gauche interaction because it is anti to C3 and C5.
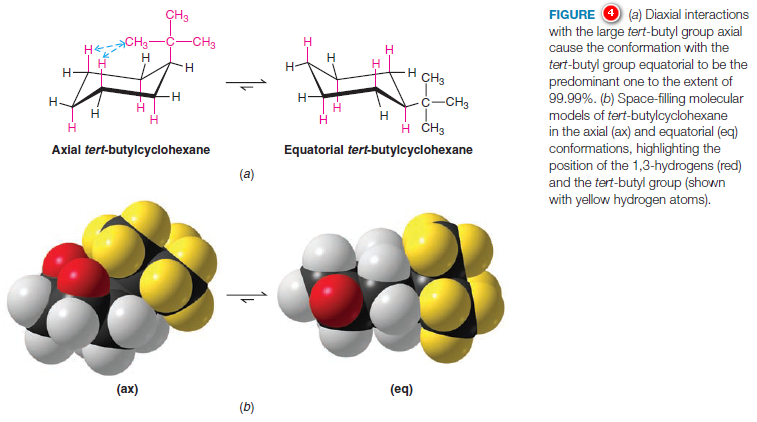
1,3-Diaxial Interactions of a tert-Butyl Group
– In cyclohexane derivatives with larger alkyl substituents, the strain caused by 1,3-diaxial interactions is even more pronounced.
– The conformation of tert-butylcyclohexane with the tert butyl group equatorial is estimated to be approximately 21 kJ mol-1 more stable than the axial form (Fig.4).
– This large energy difference between the two conformations means that, at room temperature, 99.99% of the molecules of tertbutylcyclohexane have the tert-butyl group in the equatorial position.
– The molecule is not conformationally “locked,” however; it still flips from one chair conformation to the other.
Reference: Organic chemistry / T.W. Graham Solomons, Craig B.Fryhle, Scott A. Snyder, / ( eleventh edition) / 2014.