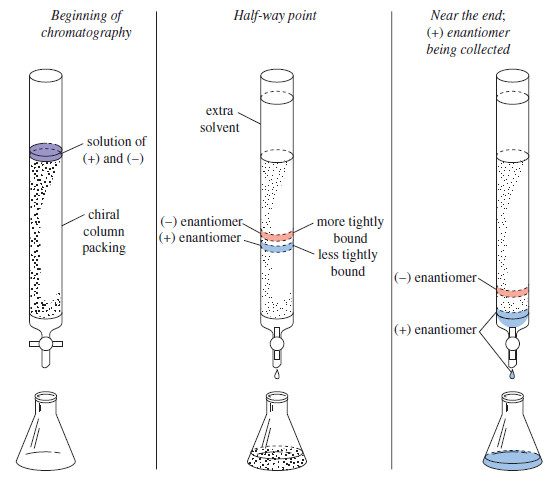
Racemic Mixtures
Racemic Mixtures
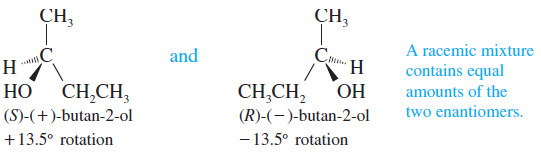
– Suppose we had a mixture of equal amounts of (+)-butan-2-ol and (-)-butan-2-ol
– The (+) isomer would rotate polarized light clockwise with a specific rotation of +13.5o ,and the isomer (-) would rotate the polarized light counterclockwise by exactly the same amount.
– We would observe a rotation of zero, just as though butan-2-ol were achiral.
– A solution of equal amounts of two enantiomers, so that the mixture is optically inactive, is called a racemic mixture.
– Sometimes a racemic mixture is called a racemate, a(±) pair, or a (d,l) pair.
– A racemic mixture is symbolized by placing (±) or (d,l) in front of the name of the compound.
– For example, racemic butan-2-ol would be symbolized by “(±)-butan-2-ol” or “(d,l)-butan-2-ol.”
– You might think that a racemic mixture would be unusual, since it requires exactly equal amounts of the two enantiomers.
– This is not the case, however. Many reactions lead to racemic products, especially when an achiral molecule is converted to a chiral molecule.
Racemic mixture in Chemical reactions
– A reaction that uses optically inactive reactants and catalysts cannot produce a product that is optically active.
– Any chiral product must be formed as a racemic mixture.
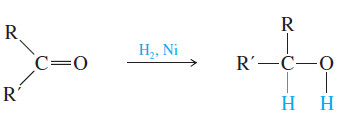
– For example, hydrogen adds across the C=O double bond of a ketone to produce an alcohol.
– Because the carbonyl group is flat, a simple ketone such as butan-2-one is achiral.
– Hydrogenation of butan-2-one gives butan-2-ol, a chiral molecule (see Figure).
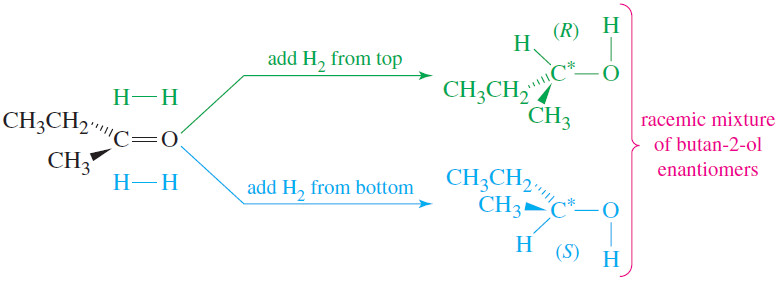
– This reaction involves adding hydrogen atoms to the C=O carbon atom and oxygen atom.
– If the hydrogen atoms are added to one face of the double bond, the (S) enantiomer results.
– Addition of hydrogen to the other face forms the (R) enantiomer.
– It is equally probable for hydrogen to add to either face of the double bond, and equal amounts of the (R) and (S) enantiomers are formed.
– Logically, it makes sense that optically inactive reagents and catalysts cannot form optically active products.
– If the starting materials and reagents are optically inactive, there is no reason for the dextrorotatory product to be favored over a levorotatory one or vice versa.
– The (+) product and the (-) product are favored equally, and they are formed in equal amounts: a racemic mixture.
Application of Racemic Mixtures in Drugs
– Many drugs currently on the market are racemic mixtures.
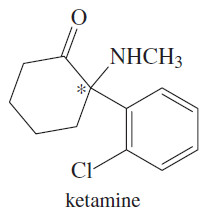
– Ketamine, for example, is a potent anesthetic agent, but its use is limited because it is hallucinogenic (making it a drug of abuse widely known as (K).
– The (S) isomer is responsible for the anesthetic effects, and the (R) isomer causes the hallucinogenic effects.