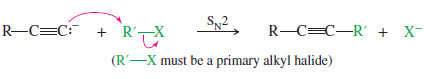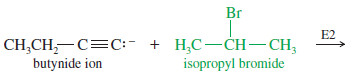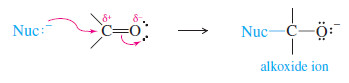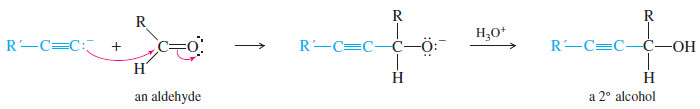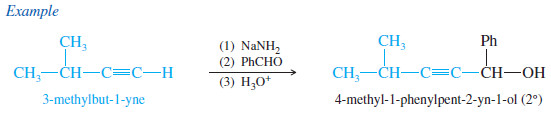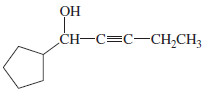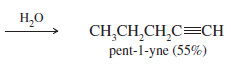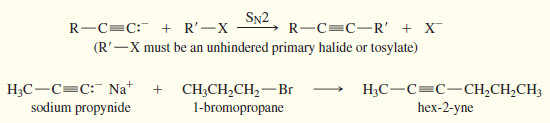Synthesis of alkynes
Synthesis of alkynes
– Two different approaches are commonly used for the synthesis of alkynes.
– In the first, an appropriate electrophile undergoes nucleophilic attack by an acetylide ion.
– The electrophile may be an unhindered primary alkyl halide (undergoes SN2), or it may be a carbonyl compound (undergoes addition to give an alcohol).
– Either reaction joins two fragments and gives a product with a lengthened carbon skeleton.
– This approach is used in many laboratory syntheses of alkynes.
– The second approach forms the triple bond by a double dehydrohalogenation of a dihalide.
– This reaction does not enlarge the carbon skeleton.
– Isomerization of the triple bond may occur, so dehydrohalogenation is useful only when the desired product has the triple bond in a thermodynamically favored position.
(1) Alkylation of Acetylide Ions
– An acetylide ion is a strong base and a powerful nucleophile.
– It can displace a halide ion from a suitable substrate, giving a substituted acetylene.
– If this SN2 reaction is to produce a good yield, the alkyl halide must be an excellent SN2 substrate: It must be methyl or primary, with no bulky substituents or branches close to the reaction center.
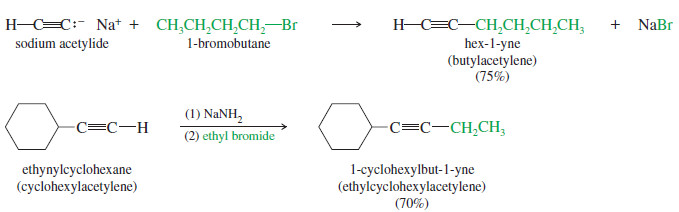
– In the following examples, acetylide ions displace primary halides to form elongated alkynes.
– If the back-side approach is hindered, the acetylide ion may abstract a proton, giving elimination by the E2 mechanism
Solved problem
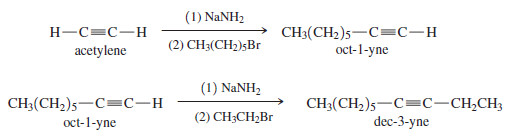
Show how to synthesize dec-3-yne from acetylene and any necessary alkyl halides.
Solution
– Another name for dec-3-yne is ethyl n-hexyl acetylene.
– It can be made by adding an ethyl group and a hexyl group to acetylene.
– This can be done in either order. We begin by adding the hexyl group.
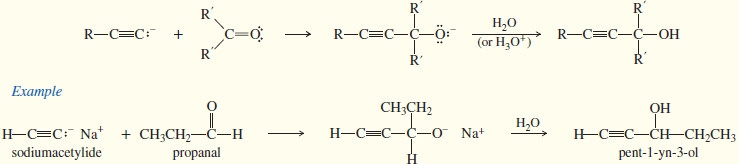
(2) Addition of Acetylide Ions to Carbonyl Groups
– Like other carbanions, acetylide ions are strong nucleophiles and strong bases.
– In addition to displacing halide ions in SN2 reactions, they can add to carbonyl (C=O) groups.
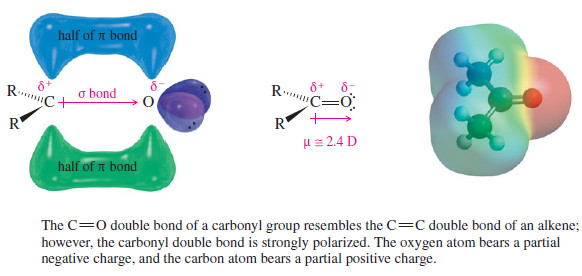
– The following figure shows the structure of the carbonyl group.
– Because oxygen is more electronegative than carbon, the (C=O) double bond is polarized.
– The oxygen atom has a partial negative charge balanced by an equal amount of positive charge on the carbon atom.
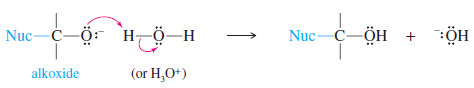
– The positively charged carbon is electrophilic; attack by a nucleophile places a negative charge on the electronegative oxygen atom.
– The product of this nucleophilic attack is an alkoxide ion, a strong base. (An alkoxide ion is the conjugate base of an alcohol, a weak acid.)
– Addition of water or a dilute acid protonates the alkoxide to give the alcohol
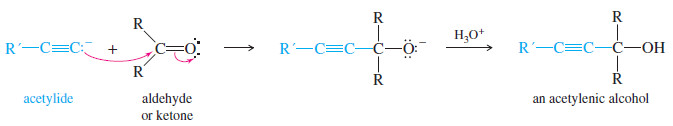
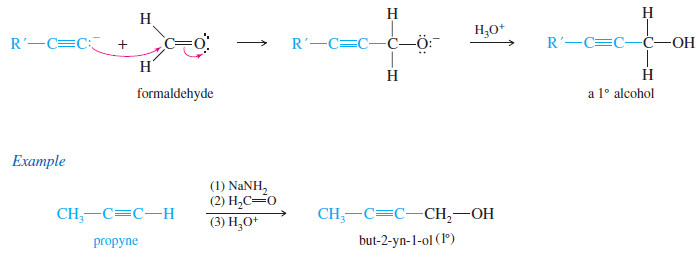
– An acetylide ion can serve as the nucleophile in this addition to a carbonyl group.
– The acetylide ion adds to the carbonyl group to form an alkoxide ion.
– Addition of dilute acid (in a separate step) protonates the alkoxide to give the alcohol.
– An acetylide adds to formaldehyde (H2C=O) to give (after the protonation step) a primary alcohol with one more carbon atom than there was in the acetylide.
– An acetylide adds to an aldehyde to give, after protonation, a secondary alcohol.
– The two groups of the secondary alcohol are the acetylide and the alkyl group that was bonded to the carbonyl group of the aldehyde
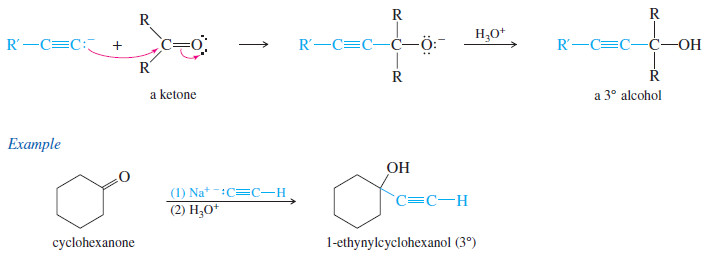
– A ketone has two alkyl groups bonded to its carbonyl carbon atom.
– Addition of an acetylide, followed by protonation, gives a tertiary alcohol.
– The three alkyl groups bonded to the carbinol carbon atom (the carbon bearing -OH the group) are the acetylide and the two alkyl groups originally bonded to the carbonyl group in the ketone.
Solved problem
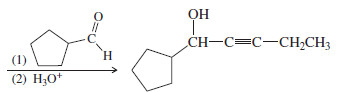
Show how you would synthesize the following compound, beginning with acetylene and any necessary additional reagents
Solution
– We need to add two groups to acetylene: an ethyl group and a six-carbon aldehyde (to form the secondary alcohol).
– If we formed the alcohol group first, the weakly acidic -OH group would interfere with the alkylation by the ethyl group.
– Therefore, we should add the less reactive ethyl group first, and add the alcohol group later in the synthesis.
– The ethyl group is not acidic, and it does not interfere with the addition of the second group:
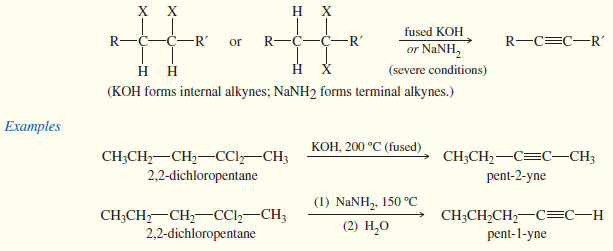
(3) Synthesis of Alkynes by Elimination Reactions
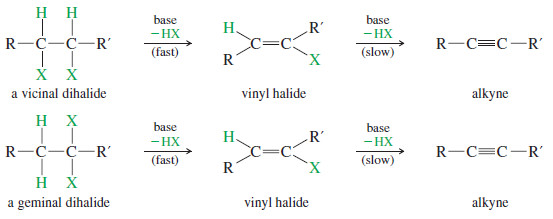
– In some cases, we can generate a carbon–carbon triple bond by eliminating two molecules of HX from a dihalide.
– Dehydrohalogenation of a geminal or vicinal dihalide gives a vinyl halide.
– Under strongly basic conditions, a second dehydrohalogenation may occur to form an alkyne
– We have already seen many examples of dehydrohalogenation of alkyl halides.
– The second step is new, however, because it involves dehydrohalogenation of a vinyl halide to give an alkyne.
– This second dehydrohalogenation occurs only under extremely basic conditions—for example, fused (molten) KOH or alcoholic KOH in a sealed tube, usually heated to temperatures close to 200 °C.
– Sodium amide is also used for the double dehydrohalogenation.
– Since the amide ion (–NH2)is a much stronger base than hydroxide, the amide reaction takes place at a lower temperature.
– Using either KOH or sodium amide at these elevated temperatures implies brutal reaction conditions, encouraging side reactions and rearrangements. Yields are often poor.
– The following reactions are carefully chosen to form products that are not prone to side reactions.
– The KOH elimination tends to give the most stable internal alkyne.
– The sodium amide elimination tends to give a terminal alkyne (where possible) because the acetylenic hydrogen is deprotonated by the amide ion, giving an acetylide ion as the initial product
Summary of Synthesis of alkynes
(1) Alkylation of acetylide ions
(2) Additions to carbonyl groups
(3) Double dehydrohalogenation of alkyl dihalides