Organic Chemistry
Organic Chemistry focuses on the structure, properties, and reactions of carbon-containing compounds. It’s essential in pharmaceuticals, polymers, and biochemistry, exploring mechanisms, functional groups, and synthesis of complex molecules.
-
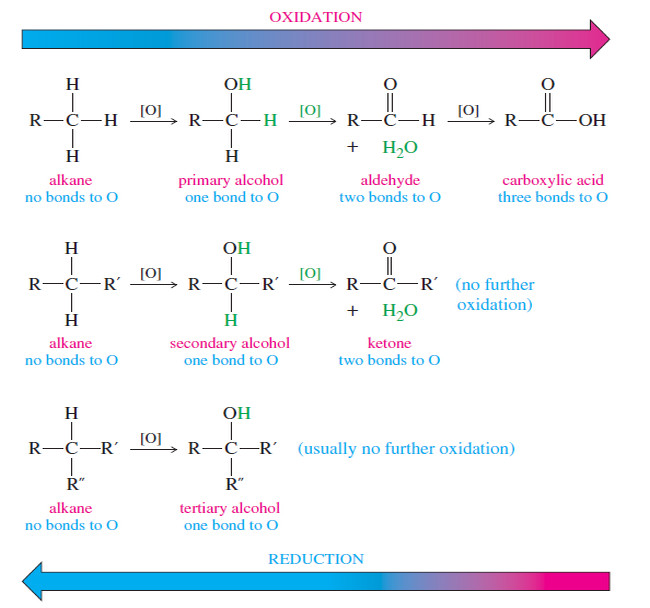
Oxidation states of Alcohols and Related Functional Groups
Oxidation states of Alcohols and Related Functional Groups – Oxidation states of Alcohols leads to ketones, aldehydes, and carboxylic acids.…
Read More » -
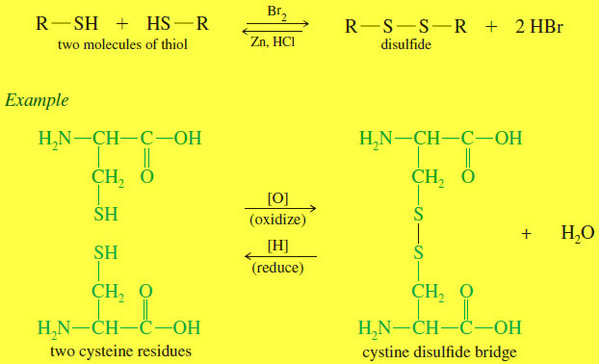
Thiols (Mercaptans)
What is Thiols? – Thiols are sulfur analogues of alcohols, with an -SH group in place of the alcohol -OH…
Read More » -
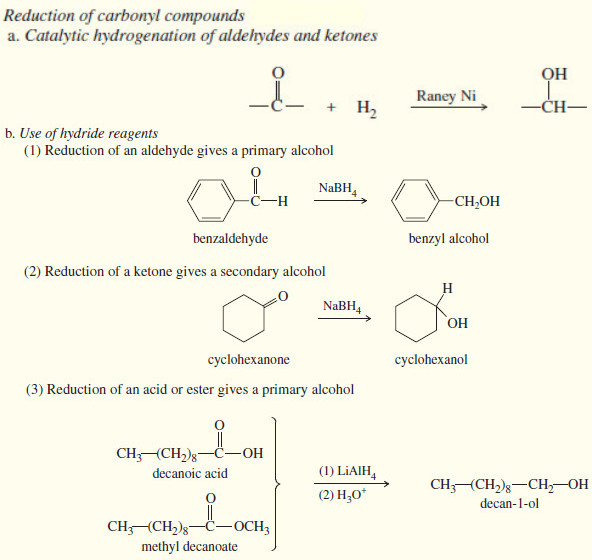
Reduction of the Carbonyl group : Synthesis of Alcohols
Reduction of the Carbonyl group : Synthesis of 1° and 2° Alcohols – Grignard reagents convert carbonyl group to alcohols…
Read More » -
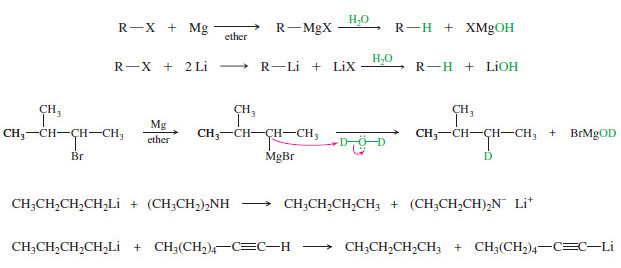
Side Reactions of Organometallic Reagents
Side Reactions of Organometallic Reagents: Reduction of Alkyl Halides – Organometallic Reagents: Grignard and organolithium reagents are strong nucleophiles and…
Read More » -
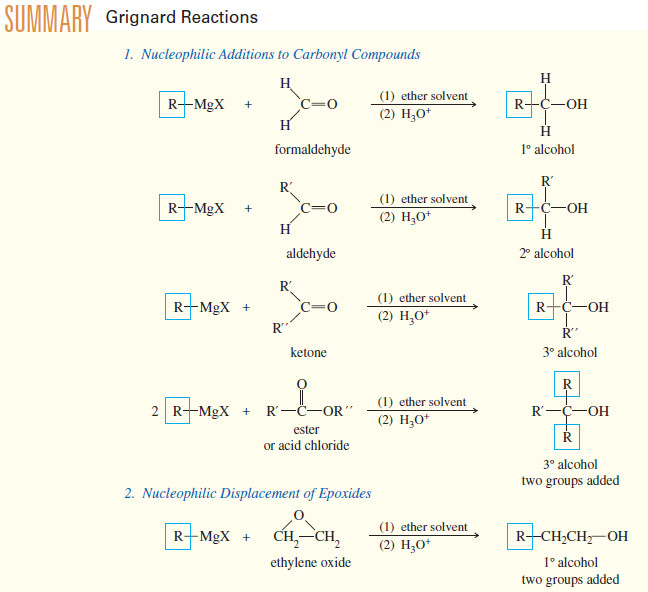
Addition of Grignard Reagents to Carbonyl Compounds
Addition of Organometallic Reagents to Carbonyl Compounds – Because they resemble carbanions, Grignard reagents and organolithium reagents are strong nucleophiles…
Read More » -
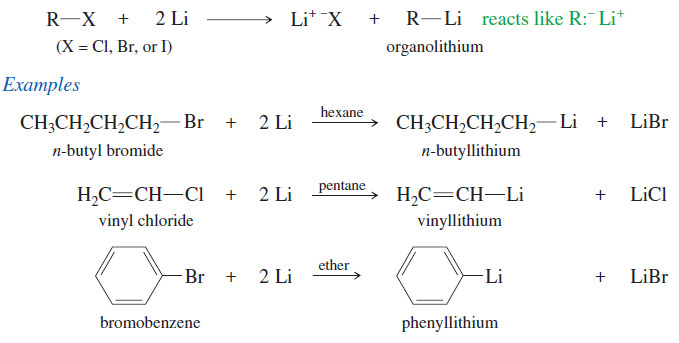
Organometallic Reagents for Alcohol Synthesis
Organometallic Reagents for Alcohol Synthesis – Organometallic compounds contain covalent bonds between carbon atoms and metal atoms. – And Organometallic…
Read More » -
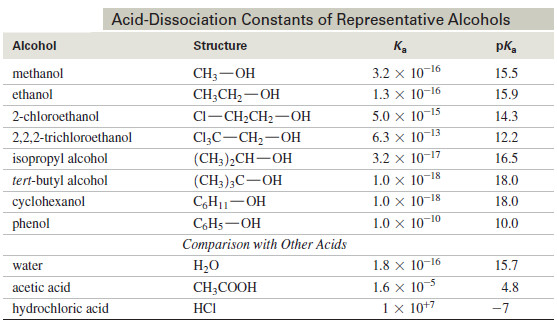
Acidity of Alcohols and Phenols
Acidity of Alcohols and Phenols – we will talk here about some Acidity of Alcohols and Phenols. – Like the…
Read More » -
Commercially Important Alcohols
we will talk about some Commercially Important Alcohols such as: Methanol , Ethanol , isopropyl alcohol (1) Commercially Important Alcohols:…
Read More » -
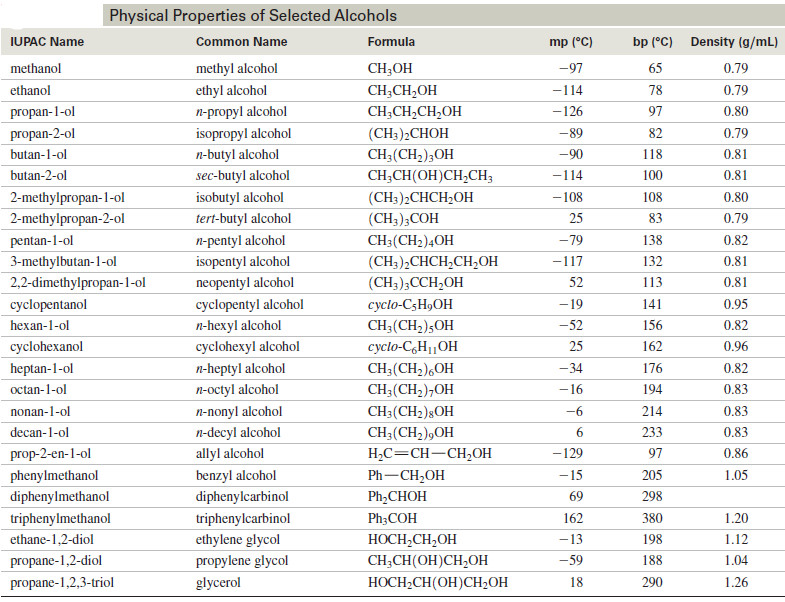
Physical Properties of Alcohols
We will discuss here Physical Properties of Alcohols: (A) Boiling Points of Alcohols (B) Solubility Properties of Alcohols Physical Properties…
Read More » -
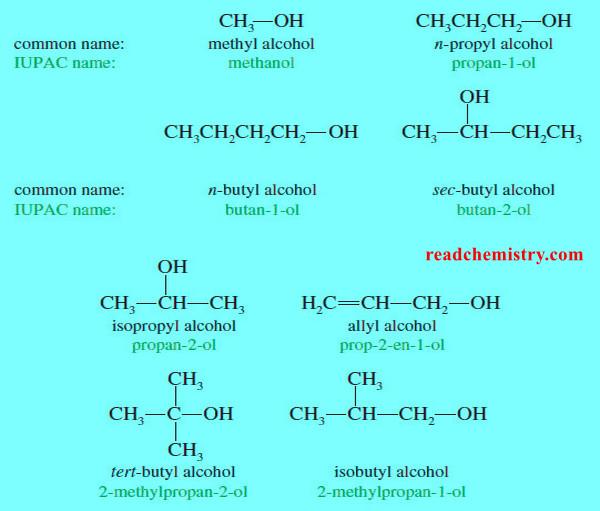
Nomenclature of Alcohols and Phenols
In this subject we will talk about Nomenclature of Alcohols and Phenols (1) Nomenclature of Alcohols: IUPAC Names – The…
Read More » -
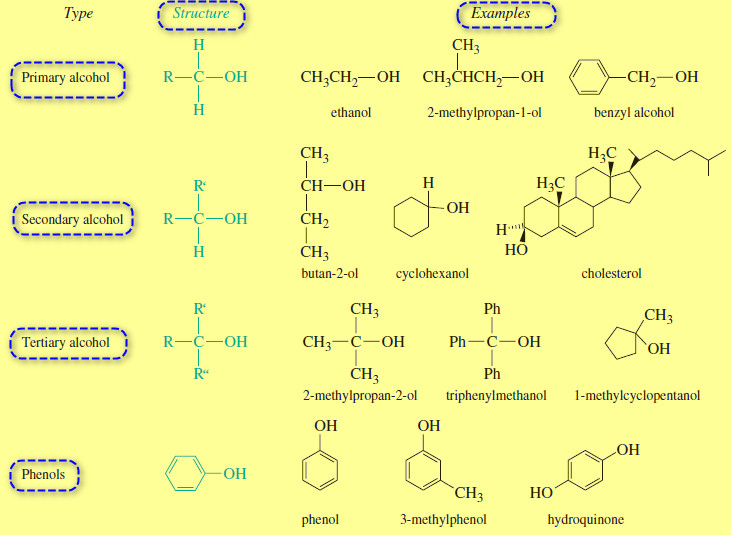
Structure and Classification of Alcohols
– In this subject we will talk about Structure and Classification of Alcohols. What are Alcohols? – Alcohols are organic…
Read More » -
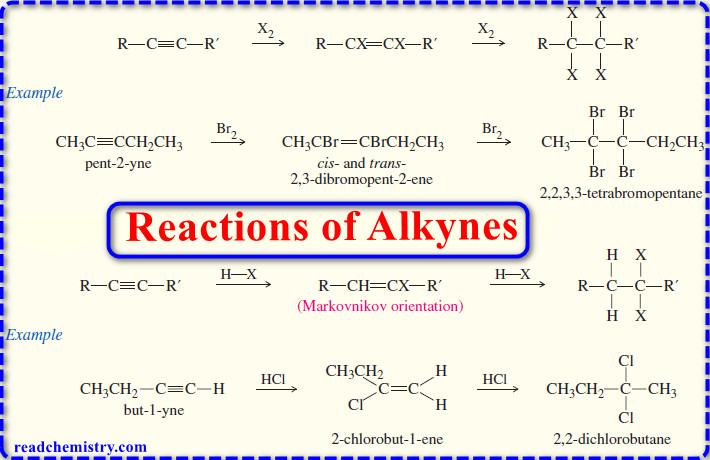
Reactions of Alkynes
Reactions of Alkynes – Many of the reactions of alkynes are similar to the corresponding reactions of alkenes because both…
Read More » -
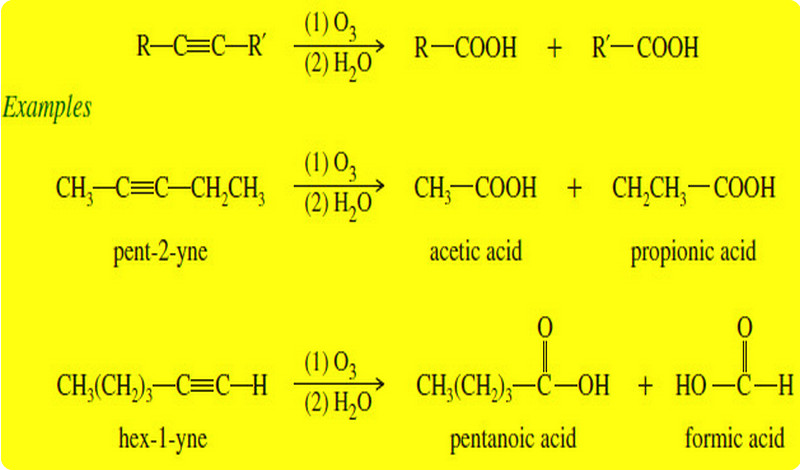
Oxidation of Alkynes
Before we discuss Oxidation of Alkynes we will talk about triple bond of Alkynes What are Alkynes? – Alkynes are…
Read More » -
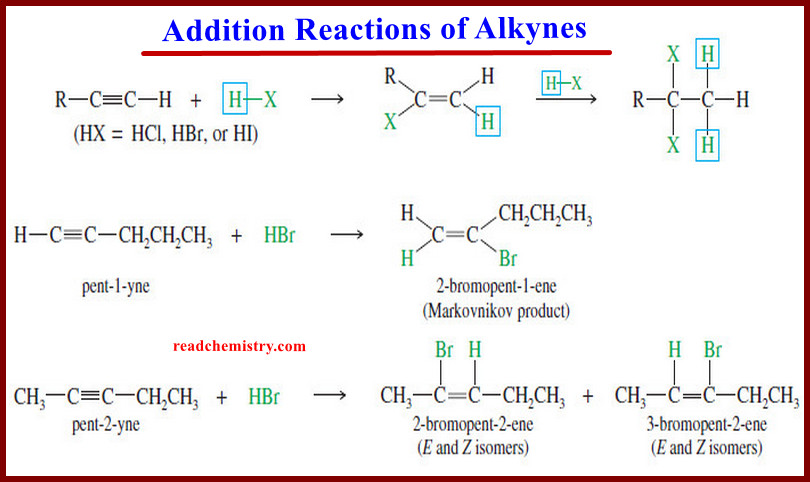
Addition Reactions of Alkynes
Addition Reactions of Alkynes – Many of the reactions of alkynes are similar to the corresponding reactions of alkenes because…
Read More » -
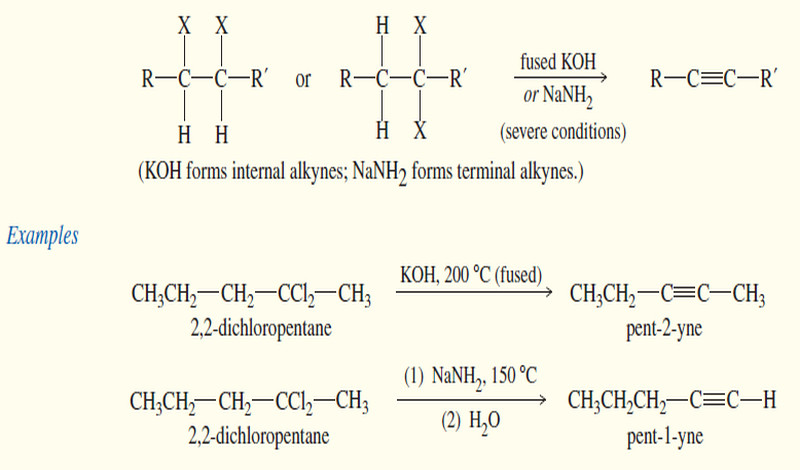
Synthesis of alkynes
Synthesis of alkynes – Two different approaches are commonly used for the synthesis of alkynes. – In the first, an…
Read More » -
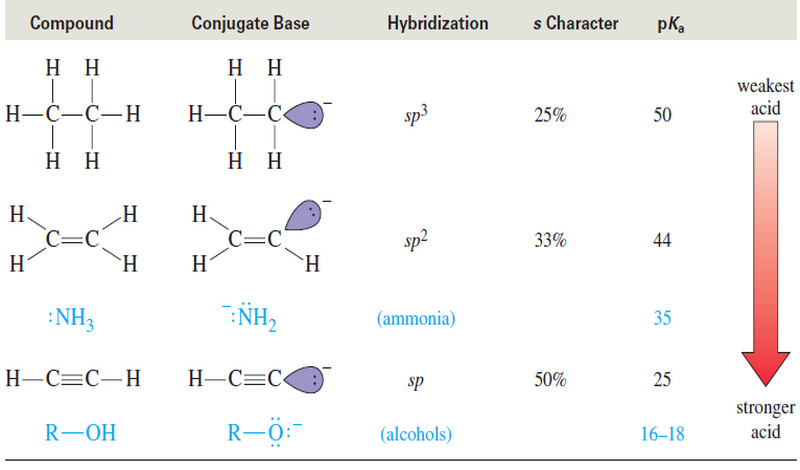
Acidity of Alkynes : Formation of Acetylide Ions
Acidity of Alkynes: Formation of Acetylide Ions – Acidity of Alkynes is the inportant factor of activity of Alkynes. –…
Read More » -
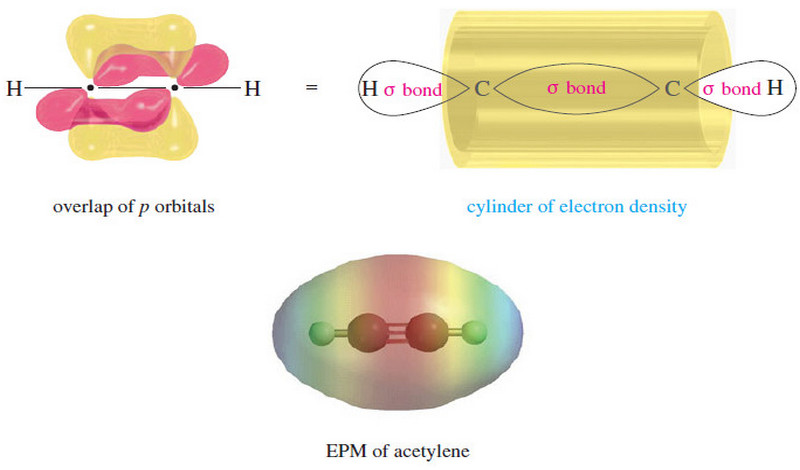
The electronic structure of Alkynes
– we studied theThe electronic structureof Alkynes (a triple bond) in This subject: The Structure of Ethyne (Acetylene): sp Hybridization…
Read More » -
Importance of Alkynes : Acetylene
Commercial Importance of Alkynes : Acetylene and Methylacetylene Uses of Acetylene and Methylacetylene – Acetylene is by far the most…
Read More » -
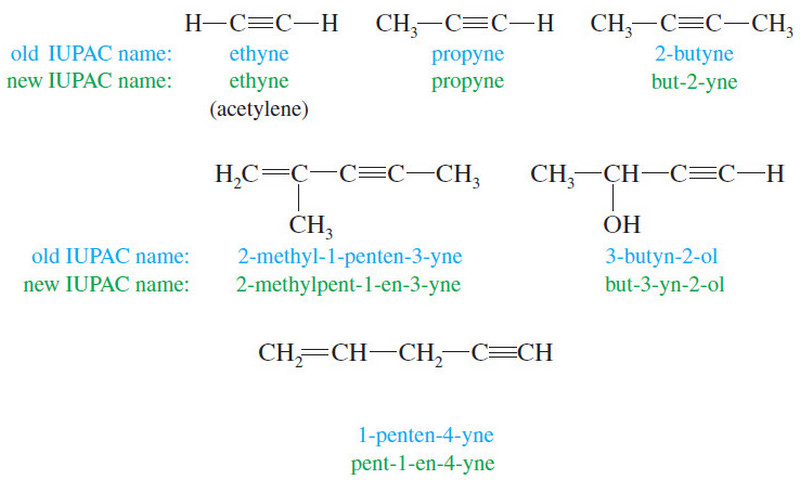
Nomenclature of Alkynes
What are Alkynes? – Alkynes are hydrocarbons that contain carbon–carbon triple bonds. – Alkynes are also called acetylenes because they…
Read More » -
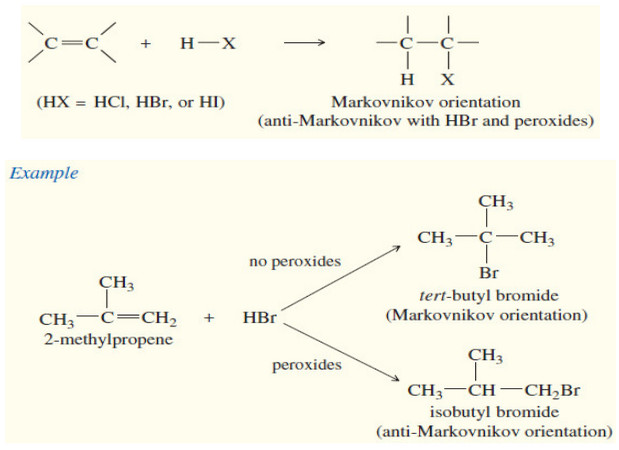
Reactions of Alkenes
– In this subject we will discuss all famous reactions of alkenes. – The reactions of alkenes include five basic…
Read More »