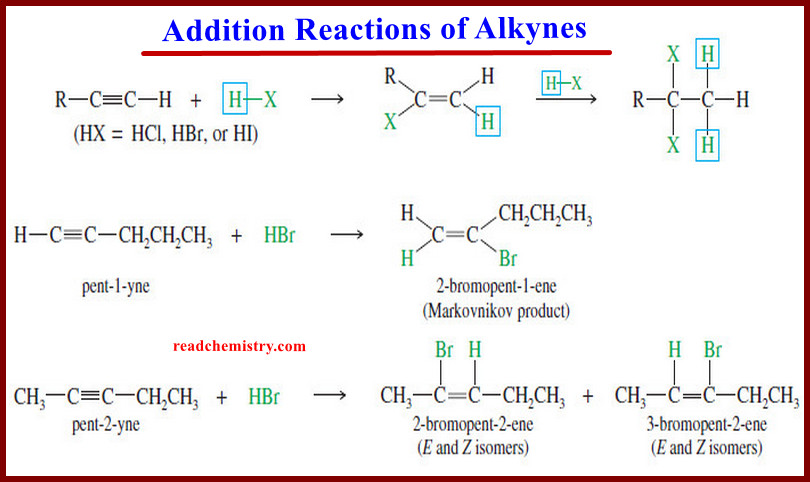
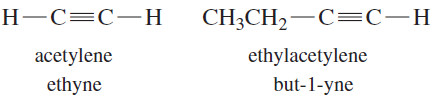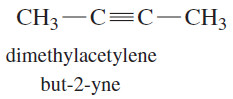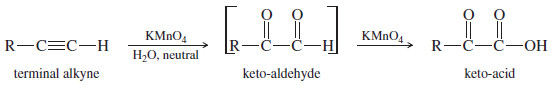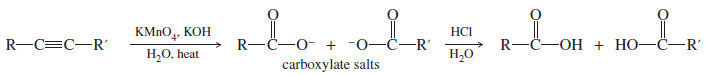Oxidation of Alkynes
Before we discuss Oxidation of Alkynes we will talk about triple bond of Alkynes
What are Alkynes?
– Alkynes are hydrocarbons that contain carbon–carbon triple bonds.
– Alkynes are also called acetylenes because they are derivatives of acetylene, the simplest alkyne
– The chemistry of the carbon–carbon triple bond is similar to that of the double bond.
– Alkynes undergo most of the same reactions as alkenes, especially the additions and the oxidations.
– Reactions that are specific to alkynes: some that depend on the unique characteristics of the C ≡ C triple bond, and others that depend on the unusual acidity of the acetylenic ≡ C – H bond.
– A triple bond gives an alkyne four fewer hydrogens than the corresponding alkane.
Oxidation of Alkynes
(1) Oxidation of Alkynes: Permanganate Oxidations
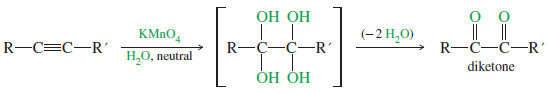
– Under mild conditions, potassium permanganate oxidizes alkenes to glycols, compounds with two -OH groups on adjacent carbon atoms.
– Recall that this oxidation involves adding a hydroxyl group to each end of the double bond (hydroxylation). A similar reaction occurs with alkynes.
– If an alkyne is treated with cold, aqueous potassium permanganate under nearly neutral conditions, an α-diketone results.
– This is conceptually the same as hydroxylating each of the two pi bonds of the alkyne, then losing two molecules of water to give the diketone.
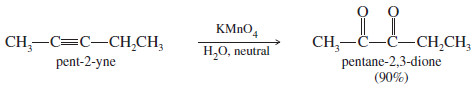
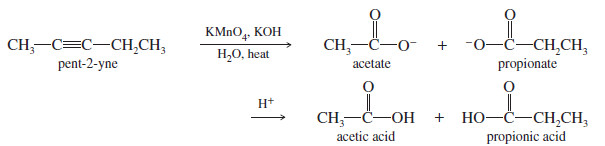
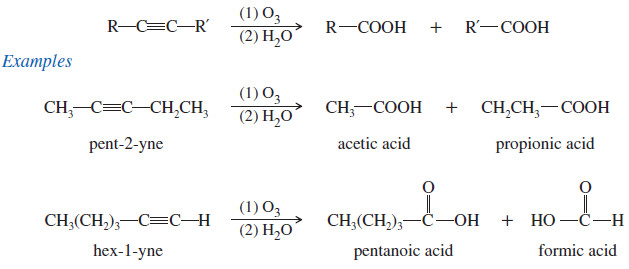
– For example, when pent-2-yne is treated with a cold, dilute solution of neutral permanganate, the product is pentane-2,3-dione.
– Terminal alkynes probably give a keto-aldehyde at first, but the aldehyde quickly oxidizes to an acid under these conditions.
– If the reaction mixture becomes warm or too basic, the diketone undergoes oxidative cleavage.
– The products are the carboxylate salts of carboxylic acids, which can be converted to the free acids by adding dilute acid.
– For example, warm, basic permanganate cleaves the triple bond of pent-2-yne to give acetate and propionate ions.
– Acidification reprotonates these anions to acetic acid and propionic acid.
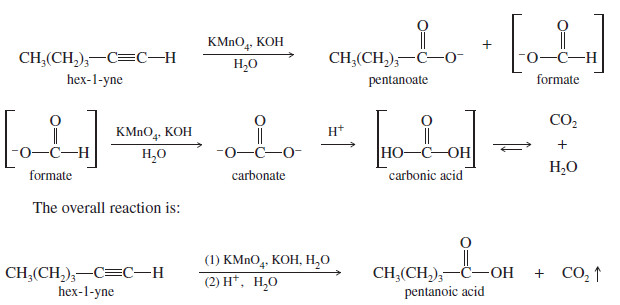
– Terminal alkynes are cleaved similarly to give a carboxylate ion and formate ion.
– Under these oxidizing conditions, formate oxidizes further to carbonate, which becomes after protonation
(2) Oxidation of Alkynes: Ozonolysis
– Ozonolysis of an alkyne, followed by hydrolysis, cleaves the triple bond and gives two carboxylic acids.
– Either permanganate cleavage or ozonolysis can be used to determine the position of the triple bond in an unknown alkyne.