Organometallic Reagents for Alcohol Synthesis
Organometallic Reagents for Alcohol Synthesis
– Organometallic compounds contain covalent bonds between carbon atoms and metal atoms.
– And Organometallic reagents are useful because they have nucleophilic carbon atoms, in contrast to the electrophilic carbon atoms of alkyl halides.
– Most metals (M) are more electropositive than carbon, and the (C-M) bond is polarized with a partial positive charge on the metal and a partial negative charge on carbon.
– The following partial periodic table shows the electronegativities of some metals used in making organometallic compounds.
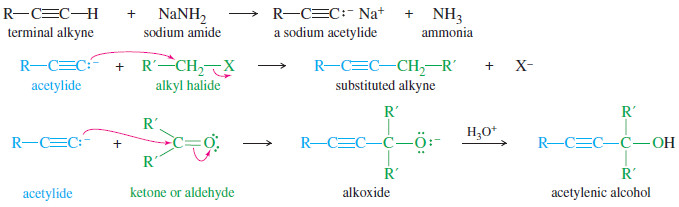
– We have already encountered one type of organometallic compound with a negative charge on carbon: sodium acetylides.
– Terminal alkynes are weakly acidic, and they are converted to sodium acetylides by treatment with an unusually strong base, sodium amide.
– These sodium acetylides are useful nucleophiles, reacting with alkyl halides and carbonyl compounds to form new carbon–carbon bonds.
– Most alkyl and alkenyl groups are not acidic enough to be deprotonated by sodium amide, but they can be made into Grignard reagents and organolithium reagents.
– These reagents are extremely versatile, providing some of our best ways of forming carbon–carbon bonds.
(A) Grignard Reagents
– Organometallic compounds of lithium and magnesium are frequently used for the synthesis of alcohols.
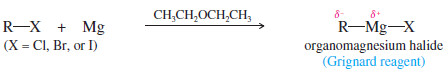
– The organomagnesium halides, of empirical formula (R-Mg-X) are called Grignard reagents in honor of the French chemist Victor Grignard, who discovered their utility around 1905 and received the Nobel Prize in Chemistry in 1912.
– Grignard reagents result from the reaction of an alkyl halide with magnesium metal.
– This reaction is always carried out in a dry (anhydrous) ether solvent, which is needed to solvate and stabilize the Grignard reagent as it forms.
– Although we write the Grignard reagent as (R-Mg-X), the actual species in solution usually contains two, three, or four of these units associated together with several molecules of the ether solvent.
– Diethyl ether CH3CH2-O-CH2CH3 , is the most common solvent for these reactions, although other ethers are also used.
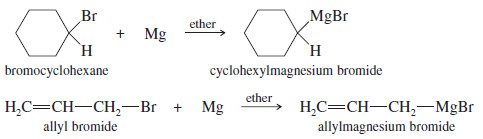
– Grignard reagents may be made from primary, secondary, and tertiary alkyl halides, as well as from vinyl and aryl halides.
– Alkyl iodides are the most reactive halides, followed by bromides and chlorides. Alkyl fluorides generally do not react.
– The following reactions show the formation of some typical Grignard reagents.
Reactivity: R-I > R-Br > R-Cl >> R-F
– The following reactions show the formation of some typical Grignard reagents.
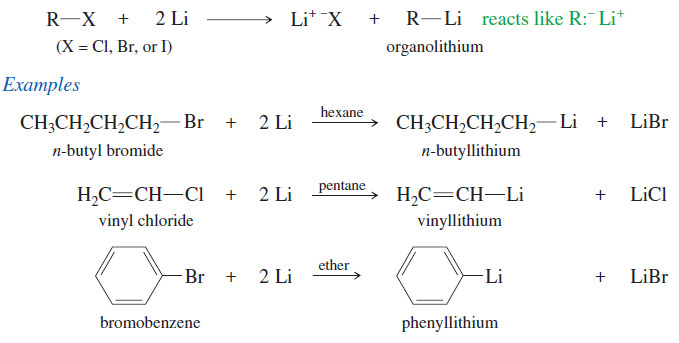
(B) Organolithium Reagents
– Like magnesium, lithium reacts with alkyl halides, vinyl halides, and aryl halides to form organometallic compounds.
– Ether is not necessary for this reaction.
– Organolithium reagents are made and used in a wide variety of solvents, including alkanes.
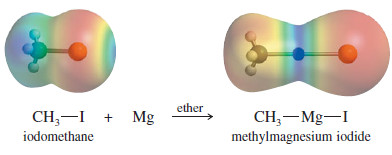
– The electrostatic potential map (EPM) of methyllithium is shown at left.
– The blue (electron-poor) color of the metal results from its partial positive charge, and the red (electron-rich) color of the methyl group shows its partial negative charge.