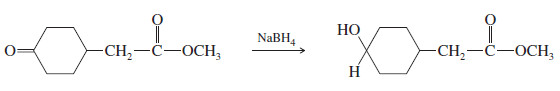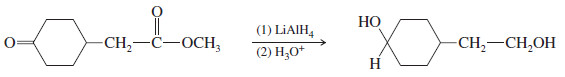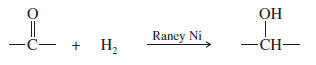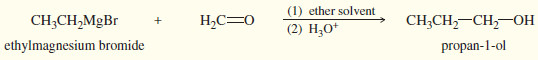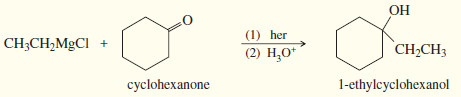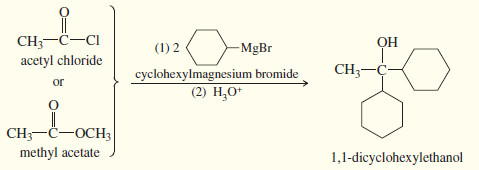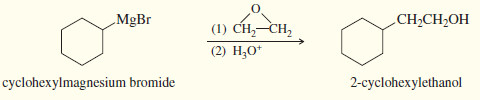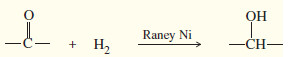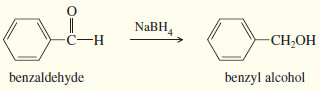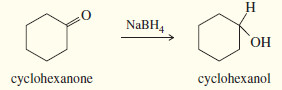Reduction of the Carbonyl group : Synthesis of Alcohols
Reduction of the Carbonyl group : Synthesis of 1° and 2° Alcohols
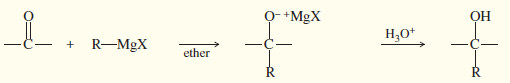
– Grignard reagents convert carbonyl group to alcohols by adding alkyl groups.
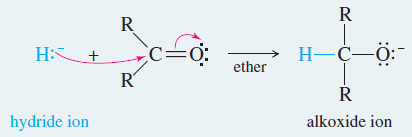
– Hydride reagents add a hydride ion (H:–) reducing the carbonyl group to an alkoxide ion with no additional carbon atoms.
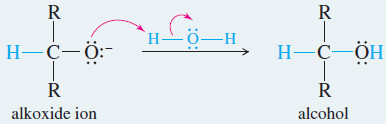
– Subsequent protonation gives the alcohol.
– Converting a ketone or an aldehyde to an alcohol involves adding two hydrogen atoms across the C=O bond: a reduction. Mechanism under shows the mechanism for this reduction.
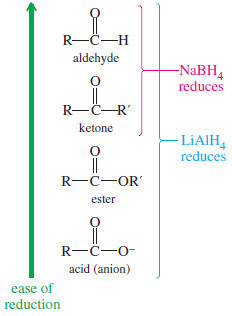
– The two most useful hydride reagents, sodium borohydride (NaBH4) and lithium aluminum hydride (LiAlH4) reduce carbonyl groups in excellent yields.
– These reagents are called complex hydrides because they do not have a simple hydride structure such as Na+H– or Li+H– Instead, their hydrogen atoms, bearing partial negative charges, are covalently bonded to boron and aluminum atoms.
– This arrangement makes the hydride a better nucleophile while reducing its basicity.
Mechanism: Hydride Reduction of a Carbonyl Group
– Sodium borohydride and lithium aluminum hydride reduce ketones and aldehydes to alcohols.
Reaction 1: Nucleophilic attack by the hydride ion forms an alkoxide ion.
Reaction 2: After the first reaction is complete, water or dilute acid is added to protonate the alkoxide.
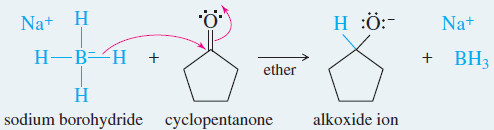
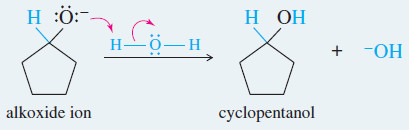
Example: Hydride reduction of cyclopentanone to cyclopentanol.
Reaction 1: Nucleophilic attack by the hydride ion forms an alkoxide ion.
Reaction 2: After the first reaction is complete, water or dilute acid is added to protonate the alkoxide.
– Aluminum is less electronegative than boron, so more of the negative charge in the ion is borne by the hydrogen atoms.
– Therefore, lithium aluminum hydride (LAH) is a much stronger reducing agent, and it is much more difficult to work with than sodium borohydride.
– LAH reacts explosively with water and alcohols, liberating hydrogen gas and sometimes starting fires.
– Sodium borohydride reacts slowly with alcohols and with water as long as the pH is high (basic).
– Sodium borohydride is a convenient and highly selective reducing agent.
(A) Uses of Sodium Borohydride
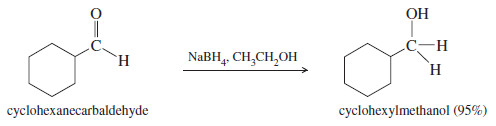
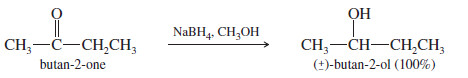
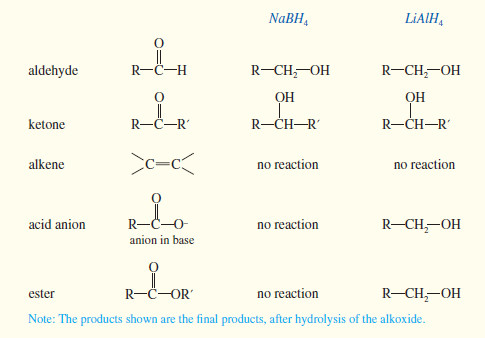
– Sodium borohydride (NaBH4) reduces aldehydes to primary alcohols, and ketones to secondary alcohols.
– The reactions take place in a wide variety of solvents, including alcohols, ethers, and water. The yields are generally excellent
– Sodium borohydride is selective; it usually does not react with carbonyl groups that are less reactive than ketones and aldehydes.
– For example, carboxylic acids and esters are unreactive toward borohydride reduction.
– Thus, sodium borohydride can reduce a ketone or an aldehyde in the presence of an acid or an ester.
(B) Uses of Lithium Aluminum Hydride
– Lithium aluminum hydride (LiAlH4 abbreviated LAH) is a much stronger reagent than sodium borohydride.
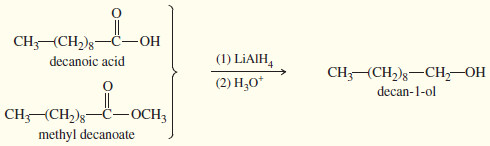
– It easily reduces ketones and aldehydes and also the less-reactive carbonyl groups: those in acids, esters, and other acid derivatives.
– LAH reduces ketones to secondary alcohols, and it reduces aldehydes, acids, and esters to primary alcohols.
– The lithium salt of the alkoxide ion is initially formed, then the (cautious!) addition of dilute acid protonates the alkoxide.
– For example, LAH reduces both functional groups of the keto ester in the previous example.
– In summary, sodium borohydride is the best reagent for reduction of a simple ketone or aldehyde.
– Using NaBH4 we can reduce a ketone or an aldehyde in the presence of an acid or an ester, but we do not have a method (so far) for reducing an acid or an ester in the presence of a ketone or an aldehyde.
– The sluggish acid or ester requires the use of LiAlH4, and this reagent also reduces the ketone or aldehyde.
SUMMARY: Reactions of LiAIH4 and NaBH4
(C) Catalytic Hydrogenation of Ketones and Aldehydes
– Reducing a ketone or an aldehyde to an alcohol involves adding two hydrogen atoms across the C=O bond.
– This addition can be accomplished by catalytic hydrogenation, commonly using Raney nickel as the catalyst.
– Raney nickel is a finely divided hydrogen-bearing form of nickel made by treating a nickel–aluminum alloy with a strong sodium hydroxide solution.
– The aluminum in the alloy reacts to form hydrogen, leaving behind a finely divided nickel powder saturated with hydrogen.
– Raney nickel is an effective catalyst for the hydrogenation of ketones and aldehydes to alcohols.
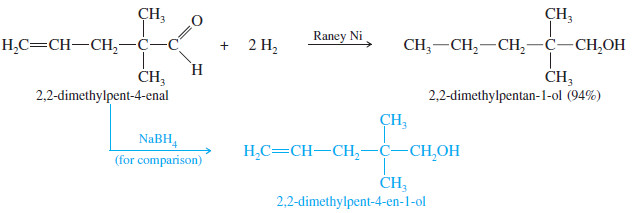
– Carbon–carbon double bonds are also reduced under these conditions, however, so any alkene double bonds in the starting material will also be reduced.
– In most cases, sodium borohydride is more convenient for reducing simple ketones and aldehydes.
Alcohol Syntheses by Nucleophilic Additions to Carbonyl groups
(A) Addition of a Grignard or organolithium reagent
(1) Addition to formaldehyde gives a primary alcohol
(2) Addition to an aldehyde gives a secondary alcohol
(3) Addition to a ketone gives a tertiary alcohol
(4) Addition to an acid halide or an ester gives a tertiary alcohol
(5) Addition to ethylene oxide gives a primary alcohol (with two additional carbon atoms added)
(B) Reduction of carbonyl group
(1) Catalytic hydrogenation of aldehydes and ketones
This method is usually not as selective or as effective as the use of hydride reagents.
(2) Use of hydride reagents
(a) Reduction of an aldehyde gives a primary alcohol
(b) Reduction of a ketone gives a secondary alcohol
(c) Reduction of an acid or ester gives a primary alcohol