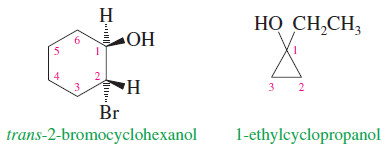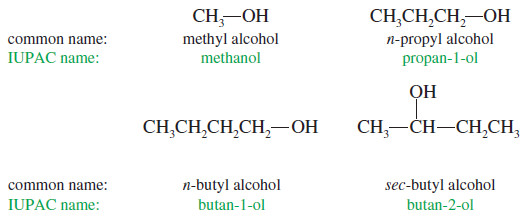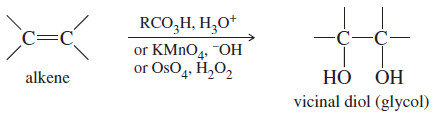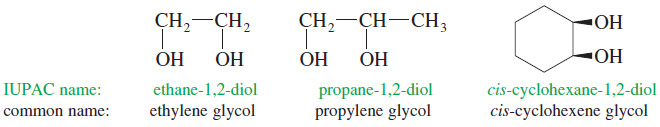Nomenclature of Alcohols and Phenols
In this subject we will talk about Nomenclature of Alcohols and Phenols
(1) Nomenclature of Alcohols: IUPAC Names
– The IUPAC system provides unique names for alcohols, based on rules that are similar to those for other classes of compounds.
– In general, the name carries the (-ol) suffix, together with a number to give the location of the hydroxyl group.
– The formal rules are summarized in the following three steps:
(1) Name the longest carbon chain that contains the carbon atom bearing the group.
– Drop the final -e from the alkane name and add the suffix -ol to give the root name.
(2) Number the longest carbon chain starting at the end nearest the hydroxyl group, and use the appropriate number to indicate the position of the -OH group. (The hydroxyl group takes precedence over double and triple bonds.)
(3) Name all the substituents and give their numbers, as you would for an alkane or an alkene.
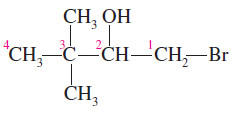
– In the following example, the longest carbon chain has four carbons, so the root name is butanol.
– The -OH group is on the second carbon atom, so this is a butan-2-ol.
– The complete IUPAC name is 1-bromo-3,3-dimethylbutan-2-ol.
– Cyclic alcohols are named using the prefix cyclo-; the hydroxyl group is assumed to be on C1.
Solved problem
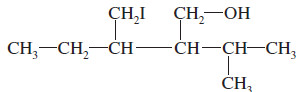
Give the systematic (IUPAC) name for the following alcohol.
Solution:
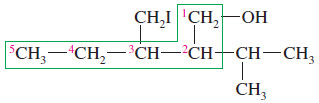
– The longest chain contains six carbon atoms, but it does not contain the carbon bonded to the hydroxyl group.
– The longest chain containing the carbon bonded to the group is the one outlined by the green box, containing five carbon atoms.
– This chain is numbered from right to left in order to give the hydroxyl-bearing carbon atom the lowest possible number.
The correct name for this compound is 3-(iodomethyl)-2-isopropylpentan-1-ol.
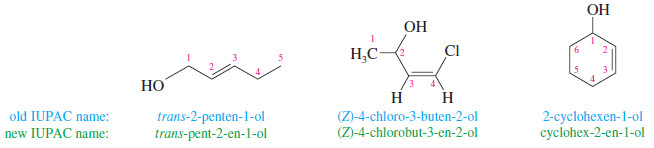
– In naming alcohols containing double and triple bonds, use the -ol suffix after the alkene or alkyne name.
– The alcohol functional group takes precedence over double and triple bonds, so the chain is numbered in order to give the lowest possible number to the carbon atom bonded to the hydroxyl group.
– The position of the -OH group is given by putting its number before the -ol suffix.
– Numbers for the multiple bonds were once given early in the name, but the 1993 revision of the IUPAC rules puts them next to the -en or -yn suffix they describe.
– Both the new and old placements of the numbers are shown in the following figure.
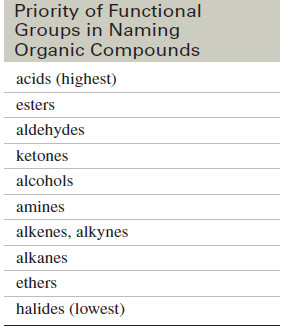
The following table is a partial table showing the order of precedence of functional groups for assigning IUPAC names.
– In general, the highest-priority functional group is considered the “main” group, and the others are treated as substituents.
– The -OH functional group is named as a hydroxy substituent when it appears on a structure with a higher-priority functional group or when the structure is too difficult to name as a simple alcohol
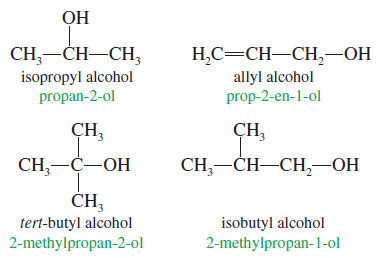
(2) Nomenclature of Alcohols: Common Names
– The common name of an alcohol is derived from the common name of the alkyl group and the word alcohol.
– This system pictures an alcohol as a molecule of water with an alkyl group replacing one of the hydrogen atoms.
– If the structure is complex, the common nomenclature becomes awkward, and the IUPAC nomenclature should be used.
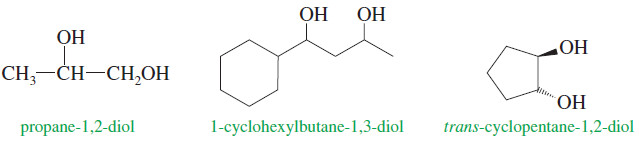
(3) Names of Diols
– Alcohols with two -OH groups are called diols or glycols.
– They are named like other alcohols except that the suffix diol is used and two numbers are needed to tell where the two hydroxyl groups are located.
– This is the preferred, systematic (IUPAC) method for naming diols.
– The term glycol generally means a 1,2-diol, or vicinal diol, with its two hydroxyl groups on adjacent carbon atoms.
– Glycols are usually synthesized by the hydroxylation of alkenes, using peroxyacids, osmium tetroxide, or potassium permanganate .
– This synthesis of glycols is reflected in their common names.
– The glycol is named for the alkene from which it is synthesized:
– The common names of glycols can be awkward and confusing because the -ene portion of the name implies the presence of an alkene double bond, but the glycol does not contain a double bond.
– We will generally use the IUPAC “diol” nomenclature for diols, but be aware that the names “ethylene glycol” (automotive antifreeze) and “propylene glycol” (used in medicines and foods) are universally accepted for these common diols.
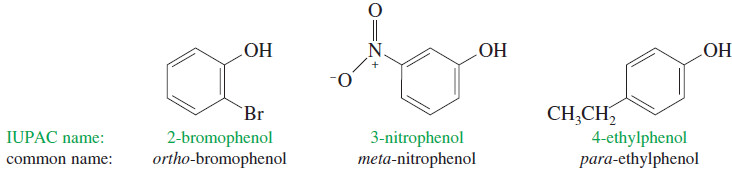
(4) Nomenclature of Phenols
– Because the phenol structure involves a benzene ring, the terms ortho (1,2-disubstituted), meta (1,3-disubstituted), and para (1,4-disubstituted) are often used in the common names.
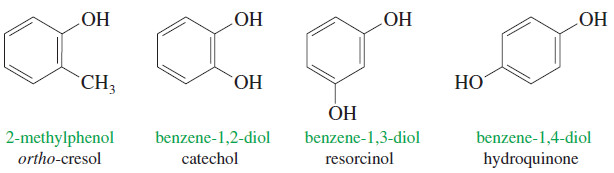
– The following examples illustrate the systematic names and the common names of some simple phenols.
– The methylphenols are called cresols, while the names of the benzenediols are based on their historical uses and sources rather than their structures.
– We will generally use the systematic names of phenolic compounds.