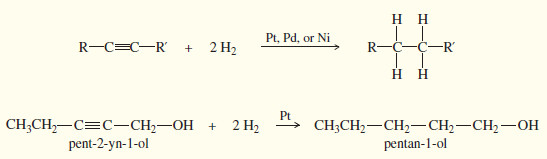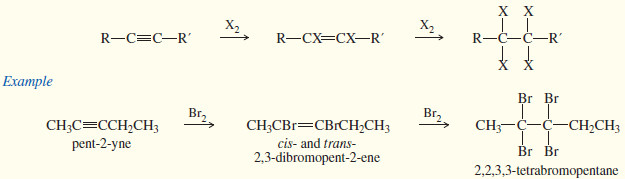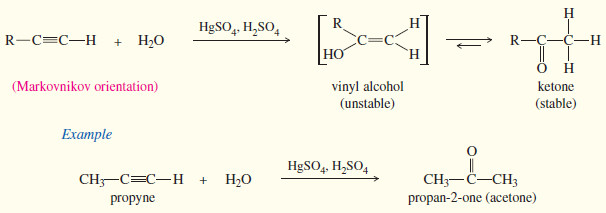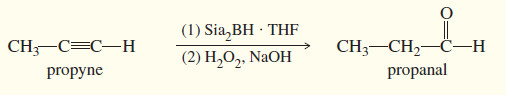Reactions of Alkynes
Reactions of Alkynes
– Many of the reactions of alkynes are similar to the corresponding reactions of alkenes because both involve pi bonds between two carbon atoms.
– Like the pi bond of an alkene, the pi bonds of an alkyne are electron-rich, and they readily undergo addition reactions.
(A) Reactions of Alkynes: Acetylide Chemistry
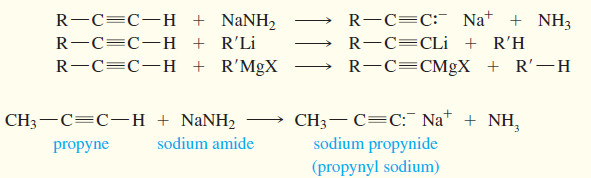
(1) Formation of acetylide anions (alkynides)
– Acidity of Alkynes is the inportant factor of activity of Alkynes.
– Terminal alkynes are much more acidic than other hydrocarbons.
– Removal of an acetylenic proton forms an acetylide ion, which plays a central role in alkyne chemistry.
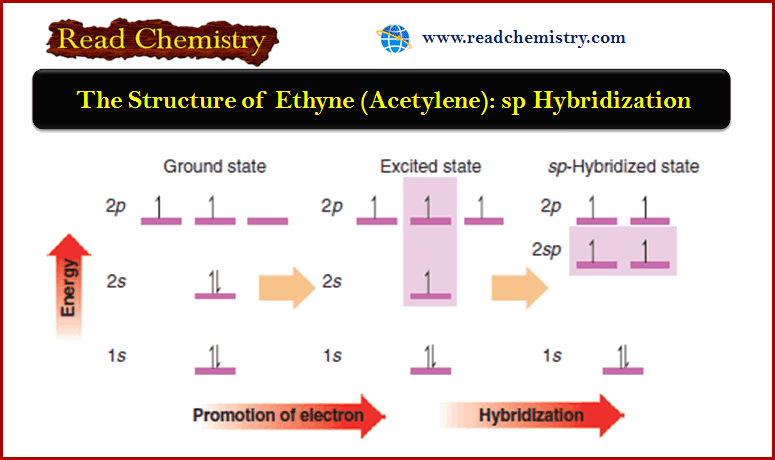
– The acidity of an acetylenic hydrogen stems from the nature of the sp hybrid ≡ C-H bond.
– Sodium amide (Na+ –NH2) is frequently used as the base in forming acetylide salts.
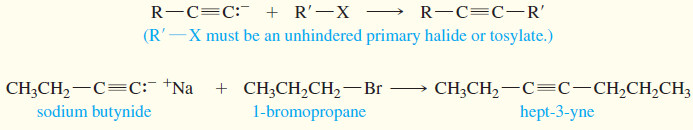
(2) Alkylation of acetylide ions
– An acetylide ion is a strong base and a powerful nucleophile.
– It can displace a halide ion from a suitable substrate, giving a substituted acetylene.
– If this SN2 reaction is to produce a good yield, the alkyl halide must be an excellent SN2 substrate: It must be methyl or primary, with no bulky substituents or branches close to the reaction center.
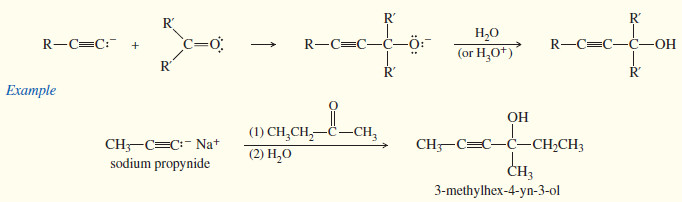
(3) Reactions with carbonyl groups
– Like other carbanions, acetylide ions are strong nucleophiles and strong bases.
– In addition to displacing halide ions in SN2 reactions, they can add to carbonyl (C=O) groups.
Because oxygen is more electronegative than carbon, the (C=O) double bond is polarized.
– The oxygen atom has a partial negative charge balanced by an equal amount of positive charge on the carbon atom.
– The positively charged carbon is electrophilic; attack by a nucleophile places a negative charge on the electronegative oxygen atom.
The product of this nucleophilic attack is an alkoxide ion, a strong base. (An alkoxide ion is the conjugate base of an alcohol, a weak acid.)
– Addition of water or a dilute acid protonates the alkoxide to give the alcohol
(B) Reactions of Alkynes: Addition to trible bond
(1) Reduction to alkanes
– In the presence of a suitable catalyst, hydrogen adds to an alkyne, reducing it to an alkane.
– For example, when either of the butyne isomers reacts with hydrogen and a platinum catalyst, the product is n-butane.
– Platinum, palladium, and nickel catalysts are commonly used in this reduction.
(2) Reduction to alkenes
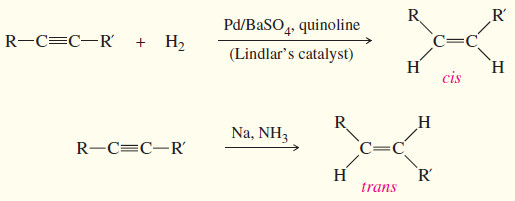
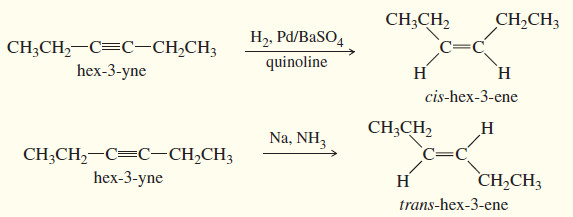
– Hydrogenation of an alkyne can be stopped at the alkene stage by using a “poisoned” (partially deactivated) catalyst made by treating a good catalyst with a compound that makes the catalyst less effective.
– Lindlar’s catalyst is a poisoned palladium catalyst, composed of powdered barium sulfate coated with palladium, poisoned with quinoline. (Equation 1)
– To form a trans alkene, two hydrogens must be added to the alkyne with anti stereochemistry.
– Sodium metal in liquid ammonia reduces alkynes with anti stereochemistry, so this reduction is used to convert alkynes to trans alkenes. (Equation 2)
(3) Addition of halogens (X2 = Cl2 , Br2)
– Bromine and chlorine add to alkynes just as they add to alkenes.
– If (1) mole of halogen adds to (1) mole of an alkyne, the product is a dihaloalkene.
– The stereochemistry of addition may be either syn or anti, and the products are often mixtures of cis and trans isomers.
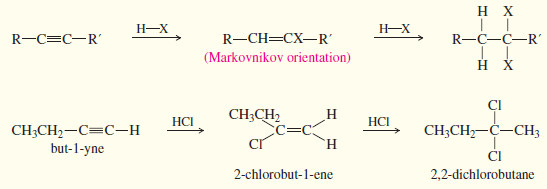
(4) Addition of hydrogen halides (where HX = HCl, HBr, or HI)
– Hydrogen halides add across the triple bond of an alkyne in much the same way they add across the alkene double bond.
– The initial product is a vinyl halide. When a hydrogen halide adds to a terminal alkyne, the product has the orientation predicted by Markovnikov’s rule.
– A second molecule of HX can add, usually with the same orientation as the first.
(5) Addition of water
(a) Catalyzed by HgSO4 / H2SO4
– Alkynes undergo acid-catalyzed addition of water across the triple bond in the presence of mercuric ion as a catalyst.
– A mixture of mercuric sulfate in aqueous sulfuric acid is commonly used as the reagent.
– The hydration of alkynes is similar to the hydration of alkenes, and it also goes with Markovnikov orientation.
– The products are not the alcohols we might expect, however.
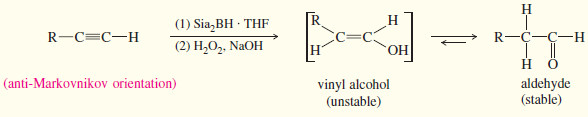
(b) Hydroboration–Oxidation
– Hydroboration–oxidation adds water across the double bonds of alkenes with anti-Markovnikov orientation.
– A similar reaction takes place with alkynes, except that a hindered dialkylborane must be used to prevent addition of two molecules of borane across the triple bond.
– Di (secondary isoamyl)borane, called “disiamylborane,” adds to the triple bond only once to give a vinylborane. (Amyl is an older common name for pentyl.)
– In a terminal alkyne, the boron atom bonds to the terminal carbon atom.
(C) Reactions of Alkynes: Oxidation of Alkynes
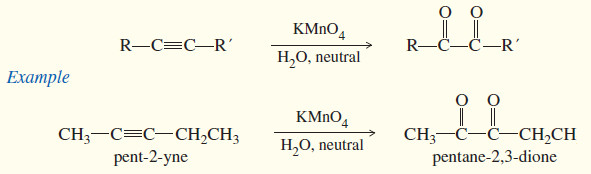
(1) Oxidation to α-diketones
– Under mild conditions, potassium permanganate oxidizes alkenes to glycols, compounds with two -OH groups on adjacent carbon atoms.
– Recall that this oxidation involves adding a hydroxyl group to each end of the double bond (hydroxylation). A similar reaction occurs with alkynes.
– If an alkyne is treated with cold, aqueous potassium permanganate under nearly neutral conditions, an α-diketone results.
– This is conceptually the same as hydroxylating each of the two pi bonds of the alkyne, then losing two molecules of water to give the diketone.
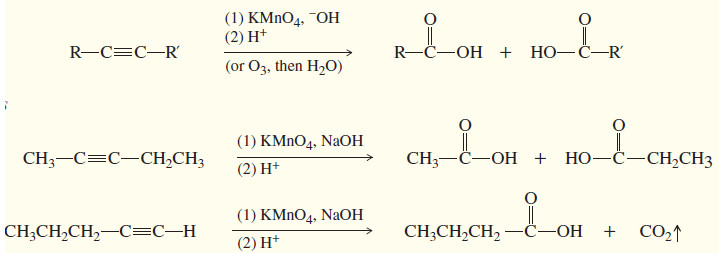
(2) Oxidative cleavage
– Ozonolysis of an alkyne, followed by hydrolysis, cleaves the triple bond and gives two carboxylic acids.
– Either permanganate cleavage or ozonolysis can be used to determine the position of the triple bond in an unknown alkyne.