Organic Chemistry
Organic Chemistry focuses on the structure, properties, and reactions of carbon-containing compounds. It’s essential in pharmaceuticals, polymers, and biochemistry, exploring mechanisms, functional groups, and synthesis of complex molecules.
-
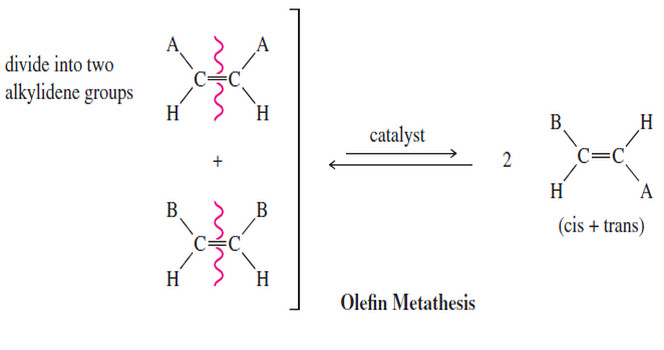
Olefin Metathesis
Olefin Metathesis – The double bond is the strongest bond in an alkene, yet it is also the most reactive…
Read More » -
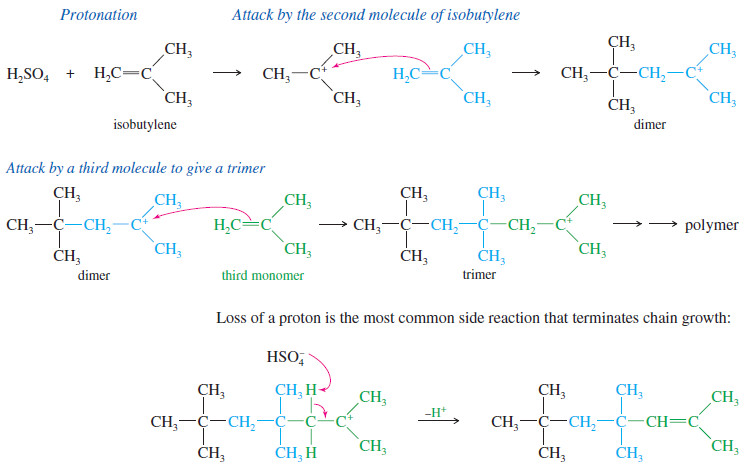
Polymerization of Alkenes
Polymerization of Alkenes – A polymer is a large molecule composed of many smaller repeating units (the monomers) bonded together.…
Read More » -
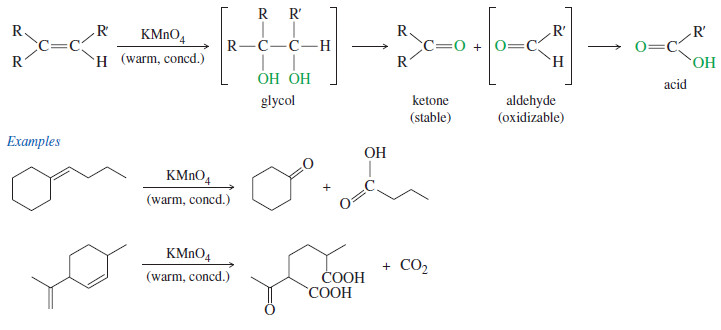
Oxidative Cleavage of Alkenes
Oxidative Cleavage of Alkenes Cleavage by Permanganate – In a potassium permanganate dihydroxylation, if the solution is warm or acidic…
Read More » -
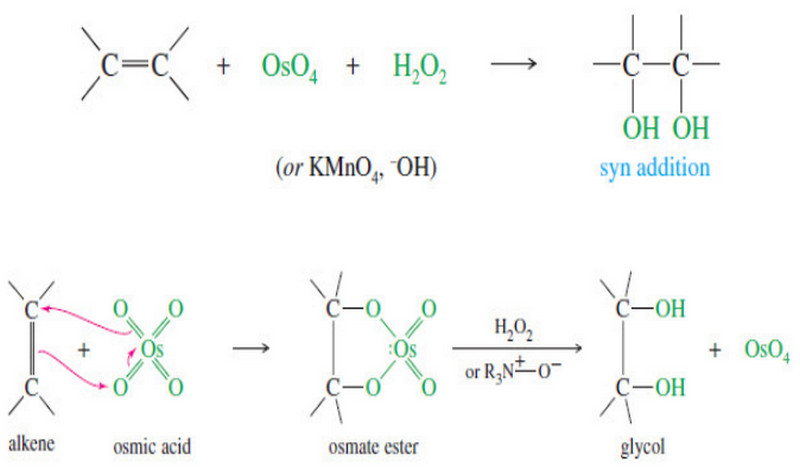
Syn Dihydroxylation of Alkenes
Syn Dihydroxylation of Alkenes – Converting an alkene to a glycol requires adding a hydroxyl group to each end of…
Read More » -
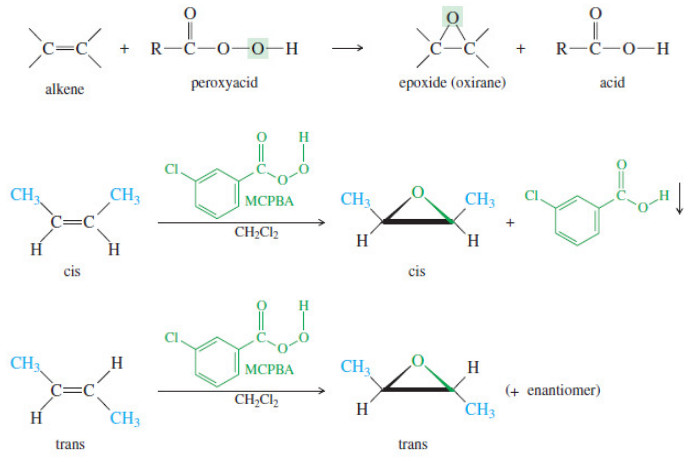
Epoxidation of Alkenes
Epoxidation of Alkenes – Some of the most important reactions of alkenes involve oxidation. – When we speak of oxidation,…
Read More » -
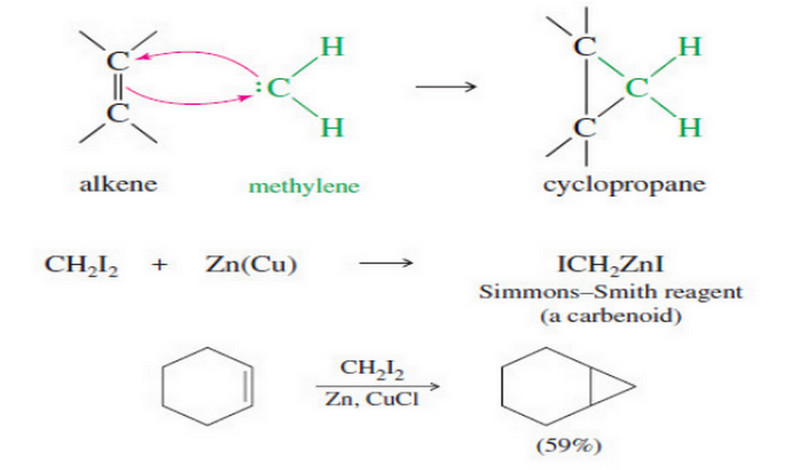
Addition of Carbenes to Alkenes
Addition of Carbenes to Alkenes – Methylene (:CH2) is the simplest of the carbenes: uncharged, reactive intermediates that have a…
Read More » -
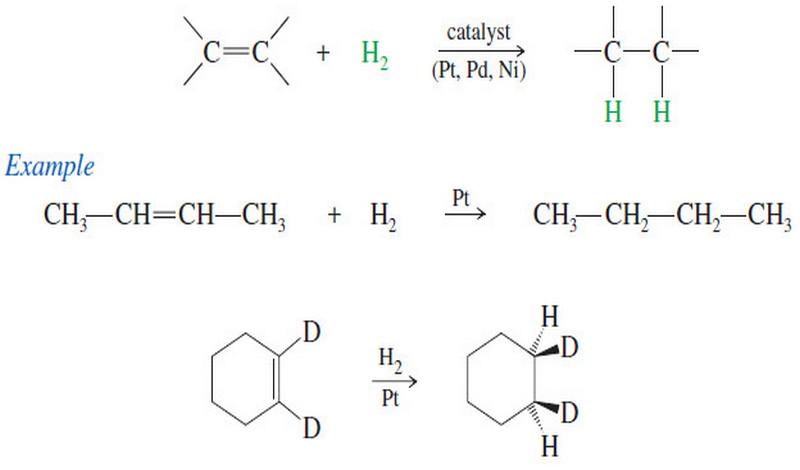
Catalytic Hydrogenation of Alkenes
Catalytic Hydrogenation of Alkenes – Although we have mentioned catalytic hydrogenation before, we now consider the mechanism and stereochemistry in…
Read More » -
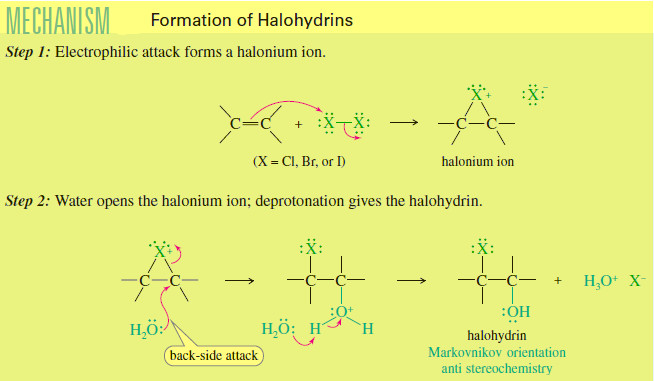
Formation of Halohydrin
Formation of Halohydrin – A halohydrin is an alcohol with a halogen on the adjacent carbon atom. – In the…
Read More » -
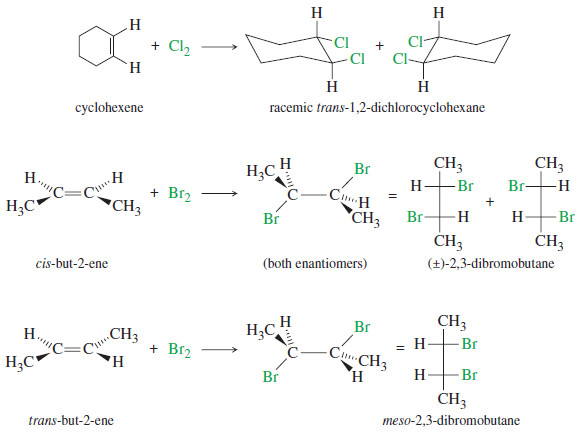
Addition of Halogens to Alkenes
Addition of Halogens to Alkenes – Addition of Halogens to Alkenes gives vicinal dihalides. (A) Mechanism of Halogen Addition –…
Read More » -
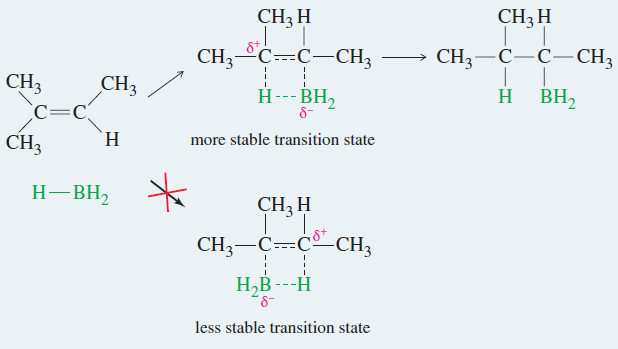
Hydroboration of Alkenes
Hydroboration of Alkenes – We have seen two methods for hydrating an alkene with Markovnikov orientation. – What if we…
Read More » -
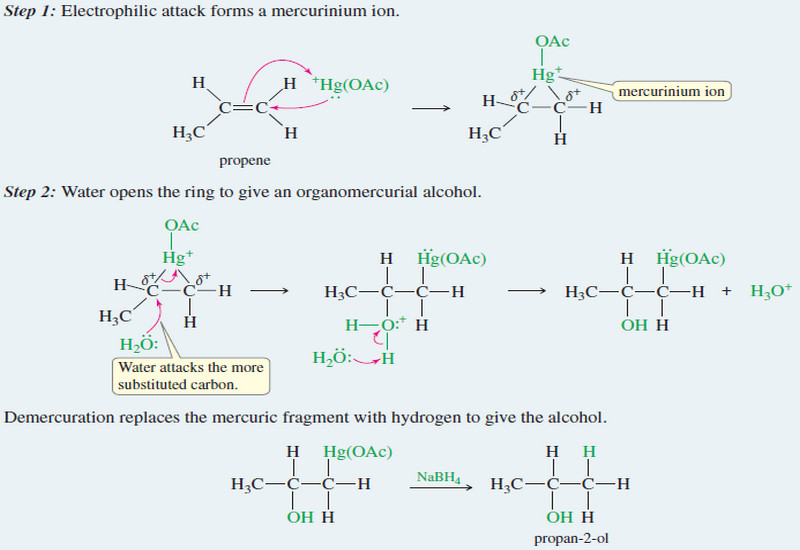
Oxymercuration–demercuration of alkenes
– Oxymercuration–demercuration of alkenes is another method for converting alkenes to alcohols with Markovnikov orientation. Hydration of alkenes by Oxymercuration–Demercuration…
Read More » -
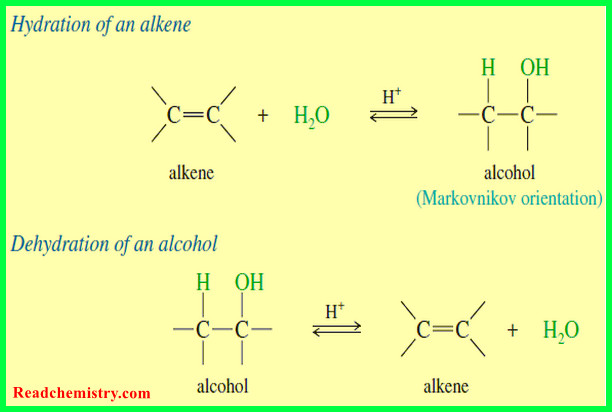
Hydration of Alkenes: Addition of Water
Hydration of Alkenes will be discussed by Addition of Water Addition of Water: Hydration of Alkenes – An alkene may…
Read More » -
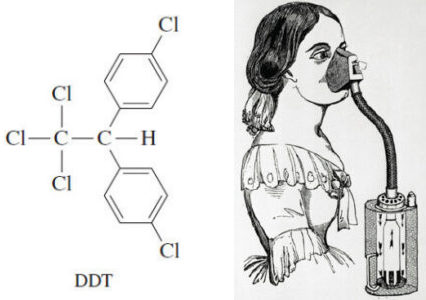
Common Uses of Alkyl Halides
Alkyl halides as Solvents – Alkyl halides are used primarily as industrial and household solvents. – Carbon tetrachloride (CCl4) was…
Read More » -
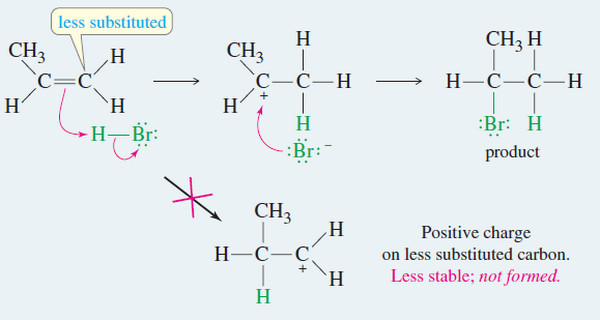
Addition of Hydrogen Halides to Alkenes: Markovnikov’s Rule
Addition of Hydrogen Halides to Alkenes : Markovnikov’s Rule Orientation of Addition: Markovnikov’s Rule – The simple mechanism shown for…
Read More » -
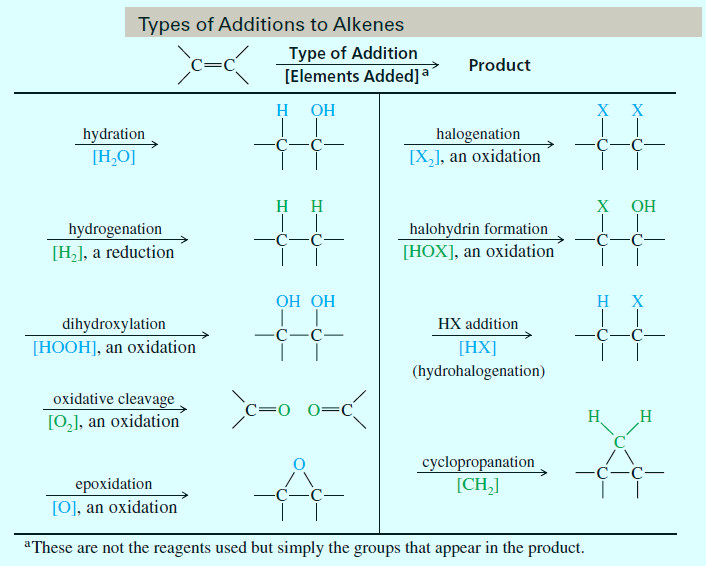
Electrophilic Addition to Alkenes
Electrophilic Addition to Alkenes Reactivity of the Carbon–Carbon Double Bond – All alkenes have a common feature: a carbon–carbon double…
Read More » -
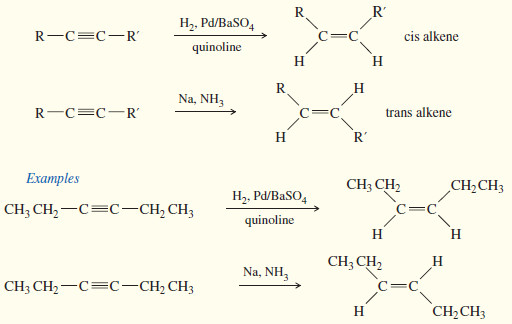
Synthesis of Alkenes – Six methods
Methods for Synthesis of Alkenes – Six methods for Synthesis of Alkenes will be discussed as follow: (1) Dehydrohalogenation of…
Read More » -
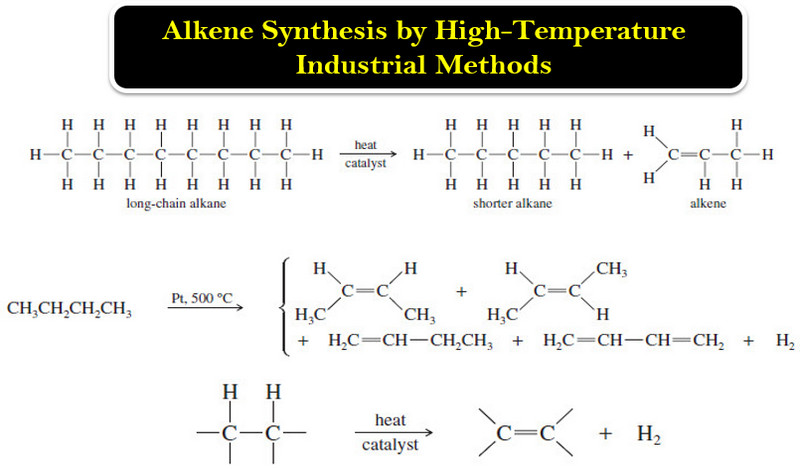
Alkene Synthesis by High-Temperature Industrial Methods
Alkene Synthesis by High-Temperature Industrial Methods (1) Catalytic Cracking of Alkanes – The least expensive way to make alkenes on…
Read More » -
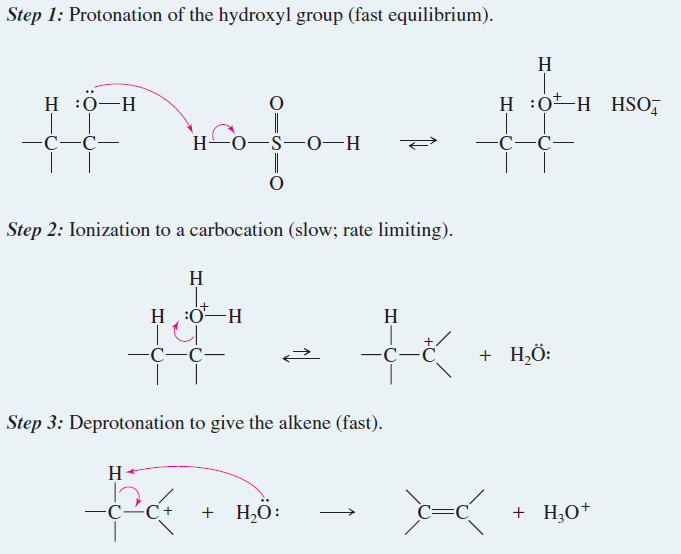
Alkene Synthesis by Dehydration of Alcohols
Alkene Synthesis by Dehydration of Alcohols – Dehydration of alcohols is a common method for making alkenes. The word dehydration…
Read More » -
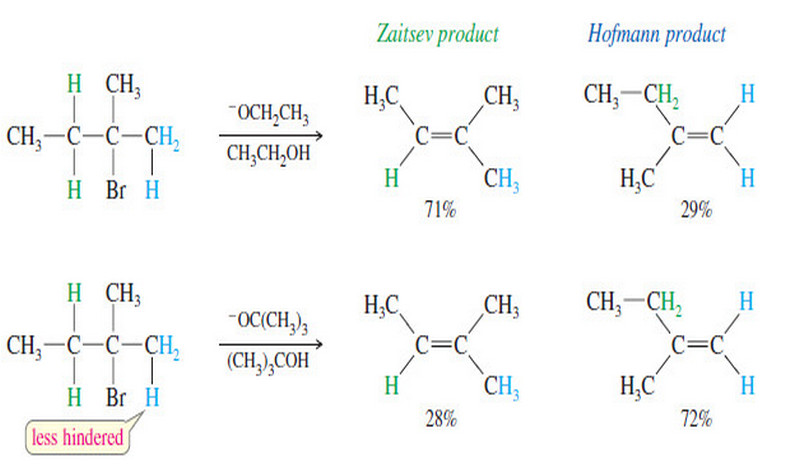
Alkene Synthesis by Elimination of Alkyl Halides
Alkene Synthesis by Elimination of Alkyl Halides – Dehydrohalogenation is the elimination of a hydrogen and a halogen from an…
Read More » -
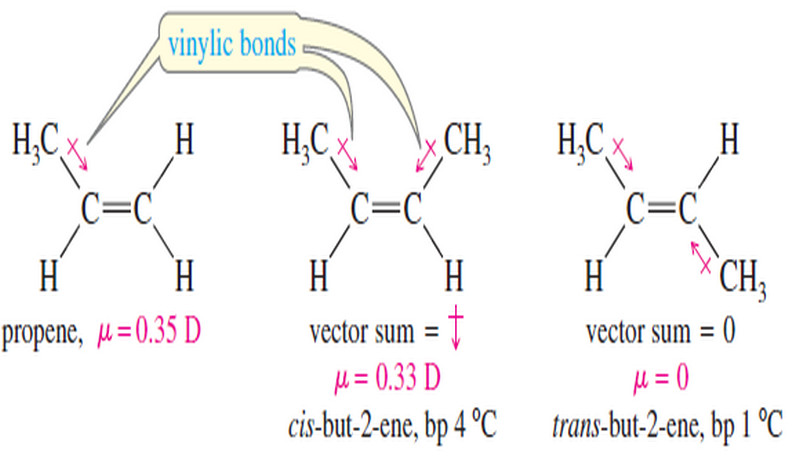
Physical Properties of Alkenes
Physical Properties of Alkenes (1) Boiling Points and Densities – Most physical properties of alkenes are similar to those of…
Read More »