Intermolecular Forces in Liquids
Intermolecular Forces in Liquids
– Intermolecular forces in liquids are collectively called van der Waals forces.
– These forces are essentially electrical in nature and result from the attraction of charges of opposite sign.
– The principal kinds of intermolecular attractions are:
(1) Dipole-dipole attractions
(2) London forces
(3) Hydrogen bonding.
– The relative size of these interactions is important so the relative effects are understood.
– Clearly, normal covalent bonds are almost 40 times the strength of hydrogen bonds.
– Covalent bonds are almost 200 times the strength of dipole-dipole forces and more than 400 times the size of London dispersion forces.
(1) Dipole–Dipole Attractions
– Dipole-dipole attractions exist between polar molecules.
– This requires the presence of polar bonds and an unsymmetrical molecule.
– These molecules have a permanent separation of positive and negative charge.
– In the illustration, the H and of HCl is permanently slightly positive charge.
– The Cl end of HCl has a permanent slight negative charge.
– The H atom in one molecule is attracted to the Cl in a neighbour.
– The intermolecular force is weak compared to a covalent bond, but this dipole-dipole interaction is one of the stronger intermolecular attractions.
(2) London Dispersion Forces
– London dispersion forces exist in nonpolar molecules.
– These forces result from temporary charge imbalances.
– The temporary charges exist because the electrons in a molecule or ion move randomly in the structure.
– The nucleus of one atom attracts electrons from the neighbouring atom.
– At the same time, the electrons in one particle repel the electrons in the neighbour and create a short-lived charge imbalance.
– These temporary charges in one molecule or atom attract opposite charges in nearby molecules or atoms.
– A local slight positive charge (δ+) in one molecule will be attracted to a temporary slight negative charge (δ–) in a neighbouring molecule.
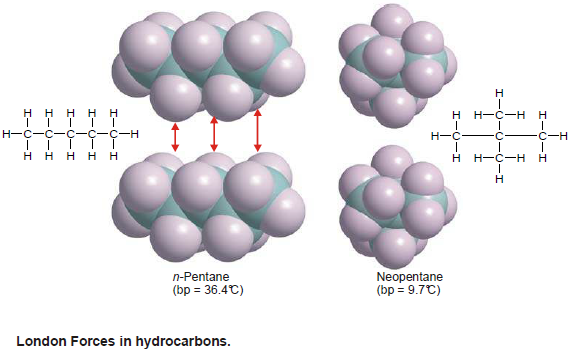
London Forces in Hydrocarbons and Organic Molecules
– The temporary separations of charge that lead to the London force attractions are what attract one nonpolar organic molecule to its neighbours.
– The possibilities for these interactions go up within creasing molecular size and surface.
– Also The larger surface increases the chances for the (induced)charge separation.
– If the molecules are linear they have more surface area than if they are folded into a sphere.
– The linear molecules have higher melting and boiling points because of the increased attractions.
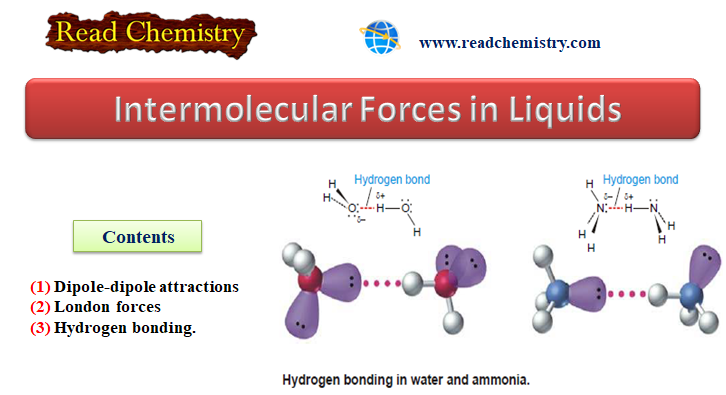
(3) Hydrogen Bonding
– Hydrogen bonding is a unique type of intermolecular attraction.
– There are two requirements:
(1) Covalent bond between an H atom and either F, O, or N. These are the three most electronegative elements.
(2) Interaction of the H atom in this kind of polar bond with a lone pair of electrons on a nearby atom like F, O, or N.

– The normal boiling point for water is 100ºC.
– The observed boiling point is high compared to the expected value.
– The predicted boiling point from the trend of boiling points for H2Te, H2Se, H2S and H2O is very low.
– If the trend continued the predicted boiling point would be below –62ºC. The (anomalous) boiling point for water is the result of hydrogen bonding between water molecules.
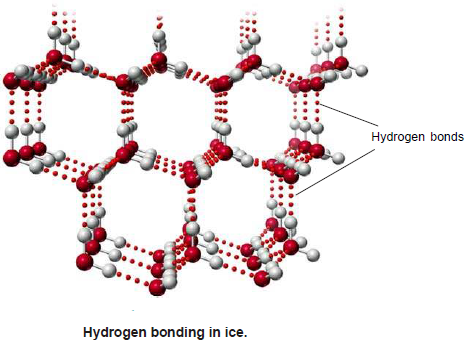
– Hydrogen bonding is responsible for the expansion of water when it freezes.
– The water molecules in the solid state have a tetrahedral arrangement for the two lone pairs and two single bonds radiating out from the oxygen.
– The lone pairs on the (O) atom can be attracted to nearby water molecules through hydrogen bonds. A cage-like structure results.
Summary of Types of Intermolecular Forces