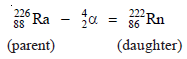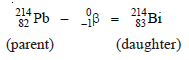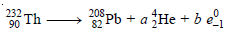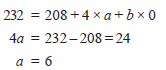Radioactive decay: Definition, Types, Examples
– In this subject, we will discuss the Radioactive decay: Definition, Types, Examples
Types of Radioactive Decay
– According to the theory put forward by Rutherford and Soddy (1903), radioactivity is a nuclear property.
– The nucleus of a radioactive atom is unstable.
– It undergoes decay or disintegration by spontaneous emission of an α- or β-particle.
– This results in the change of proton-neutron composition of the nucleus to form a more stable nucleus.
– The original nucleus is called the parent nucleus and the product is called the daughter nucleus.
– As evident from above, there are two chief types of Radioactive decay: α-decay and β-decay
α-Decay
– When a radioactive nucleus decays by the emission of an α-particle (α-emission) from the nucleus, the process is termed α-decay.
– An alpha particle has four units of atomic mass and two units of positive charge.
– If Z is the atomic number and M is the atomic mass of the parent nucleus, the daughter nucleus will have:
– Thus an α-emission reduces the atomic mass by 4 and atomic number by 2.
– For example, Radium decays by α-emission to form a new element Radon,
β-Decay
– When a radioactive nucleus decays by β-particle emission (β-emission), it is called β-decay.
– A free β-particle or electron does not exist as such in the nucleus.
– It is produced by the conversion of a neutron to a proton at the moment of emission.
– This results in the increase of one positive charge on the nucleus.
– The loss of a β-particle from the nucleus does not alter its atomic mass.
– For a parent nucleus with atomic mass M and atomic number Z, the daughter nucleus will have:
– Thus a β-emission increases the atomic number by 1 with no change in atomic mass.
– An example of β-decay is the conversion of lead-214 to bismuth-214,
– It is noteworthy that a β-emission results in the production of an isobar.
– Thus, 214Pb82 and 214Bi83 are isobaric as they have the same mass number 214 but different atomic numbers (82 and 83).
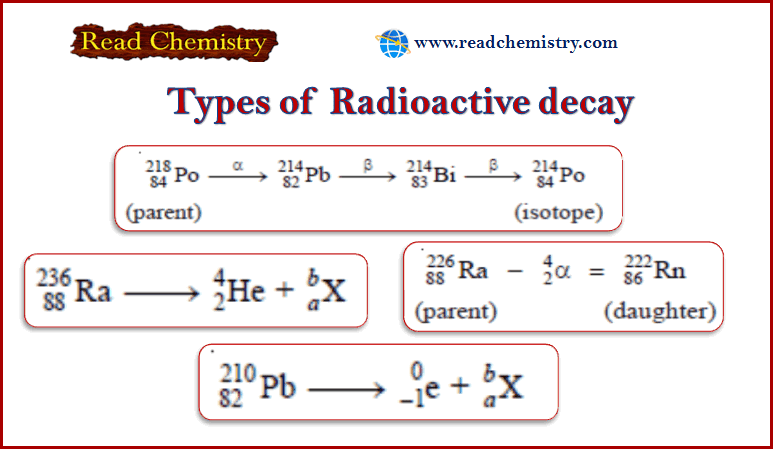
– One α-emission and two β-emissions yield an isotope.
– Let us consider the following series of changes.
– The parent element 218Po84 emits an α-particle and subsequently, two β-particles, resulting in the formation of 214Po84which is an isotope of the parent.
– Both the parent and the end-product have the same atomic number 84 but different mass numbers (218 and 214).
Solved Problems on Radioactive decay
Problem (1): How many α and β particles are emitted in passing down from 232Th90 to 208Pb82?
Solution:
– Let (a) be the number of α particles and (b) be the number of β particles emitted during the radioactive transformation.
– It can be represented as:
– Comparing the mass numbers, we get
– Comparing the atomic numbers, we get
– Substituting the value of a, we get
– Thus the number of α particles emitted = 6 and the number of β particles emitted = 4
Problem (2): 210Pb82 is a β-emitter and 226Ra88 is anα-emitter. What will be the atomic masses and atomic numbers of daughter elements of these radioactive elements? Predict the position of daughter elements in the periodic table.
Solution:
(a) 210Pb82 undergoes β-decay i.e.
– Comparing the atomic masses, we have
– and comparing the atomic numbers, we get
– Thus the daughter element will have the same atomic mass 210 and its atomic number will be 83.
– It will occupy one position right to the parent element.
(b) 236Ra88 undergoes α decay i.e.
– Comparing the atomic masses, we get:
– and comparing the atomic number, we get:
– Thus the daughter element will have an atomic mass of 232 and its atomic number will be 86.
– It will occupy two positions to the left of the parent element.
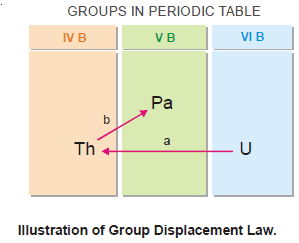
The group displacement law
– The position number of an element in a Group of the Periodic Table corresponds to its atomic number.
– If the atomic number of a given element is changed, its Group also changes accordingly.
– We know that an α-emission decreases the atomic number of the parent element by 2 and a β emission increases the atomic number by 1.
– Thus: in an α-emission, the parent element will be displaced to a Group two places to the left and in a β-emission, it will be displaced to a Group One place to the right.
– This is called the Group Displacement Law.
– It was first stated by Fajans and Soddy (1913) and is often named after them as (Fajans-Soddy Group Displacement Law).
Reference: Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolor edition.