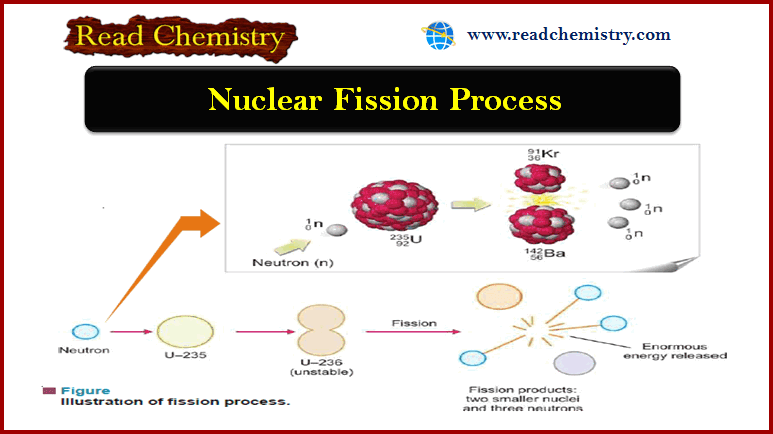
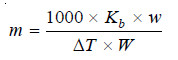Measurement of boiling point elevation
MEASUREMENT OF BOILING POINT ELEVATION
– There are several methods available for the measurement of the elevation of boiling point.
– Some of these are outlined below :
(1) Landsberger-Walker Method
This method was introduced by Landsberger and modified by Walker.
Apparatus:
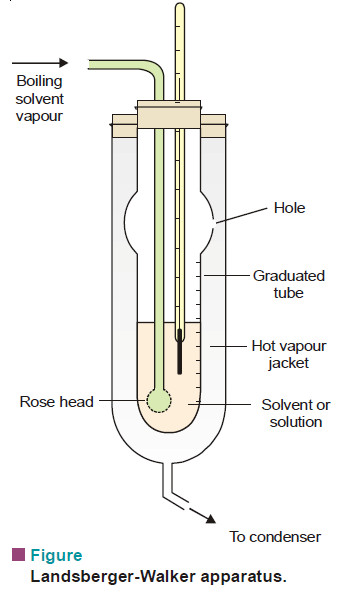
– The apparatus used in this method is shown in Fig.
– It consists of :
(i) An inner tube with a hole in its side and graduated in ml;
(ii) A boiling flask which sends solvent vapour in to the graduated tube through a ‘rosehead’ (a bulb with several holes)’
(iii) An outer tube which receives hot solvent vapour issuing from the side-hole of the inner tube;
(iv) A thermometer reading to 0.01 K, dipping in solvent or solution in the inner tube.
Procedure:
– Pure solvent is placed in the graduated tube and vapour of the same solvent boiling in a separate flask is passed into it.
– The vapour causes the solvent in the tube to boil by its latent heat of condensation.
– When the solvent starts boiling and temperature becomes constant, its boiling point is recorded.
– Now the supply of vapour is temporarily cut off and a weighed pellet of the solute is dropped into the solvent in the inner tube.
– The solvent vapour is again passed through until the boiling point of the solution is reached and this is recorded.
– The solvent vapour is then cut off, thermometer and rosehead raised out of the solution, and the volume of the solution read.
– From a difference in the boiling points of solvent and solution, we can find the molecular weight of the solute by using the expression:
where:
w = weight of solute taken,
W = weight of solvent which is given by the volume of solvent (or solution) measured in ml multiplied by the density of the solvent at its boiling point
(2) Cottrell’s Method
A method better than Landsberger-Walker method was devised by Cottrell (1910).
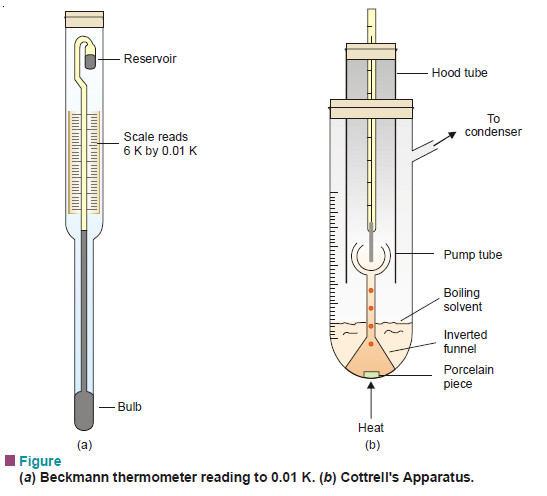
Apparatus:
– It consists of :
(i) a graduated boiling tube containing solvent or solution;
(ii) a reflux condenser which returns the vapourised solvent to the boiling tube;
(iii) a thermometer reading to 0.01 K, enclosed in a glass hood;
(iv) A small inverted funnel with a narrow stem which branches into three jets projecting at the thermometer bulb.
Beckmann Thermometer
– Beckmann Thermometer is differential thermometer(Fig.a)
– It is designed to measure small changes in temperature and not the temperature itself.
– It has a large bulb at the bottom of a fine capillary tube.
– The scale is calibrated from 0 to 6 K and subdivided into 0.01 K.
– The unique feature of this thermometer, however, is the small reservoir of mercury at the top.
– The amount of mercury in this reservoir can be decreased or increased by tapping the thermometer gently.
– In this way the thermometer is adjusted so that the level of mercury thread will rest at any desired point on the scale when the instrument is placed in the boiling (or freezing) solvent.
Procedure:
– The apparatus is fitted up as shown in Fig.(b).
-Solvent is placed in the boiling tube with a porcelain piece lying in it. It is heated on a small flame (micro burner).
– As the solution starts boiling, solvent vapour arising from the porcelain piece pump the boiling liquid into the narrow stem. Thus a mixture of solvent vapour and boiling liquid is continuously sprayed around the thermometer bulb.
– The temperature soon becomes constant and the boiling point of the pure solvent is recorded.
– Now a weighed pellet of the solute is added to the solvent and the boiling point of the solution noted as the temperature becomes steady. Also, the volume of the solution in the boiling tube is noted.
– The difference of the boiling temperatures of the solvent and solute gives the elevation of boiling point.
– While calculating the molecular weight of solute the volume of solution is converted into mass by multiplying with density of solvent at its boiling point.