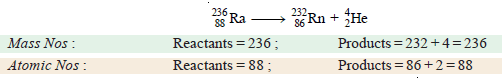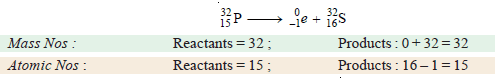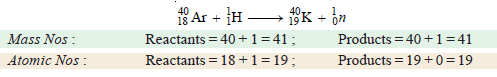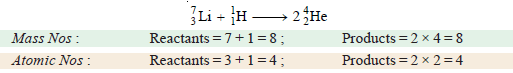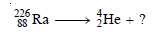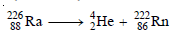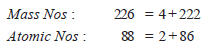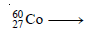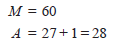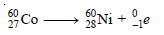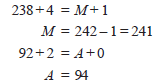Nuclear Equations: Balancing, Rules, Practice
– In this subject, we will discuss the Nuclear Equations: Balancing, Rules, Practice
Nuclear Equations
– Similar to a chemical reaction, nuclear reactions can be represented by equations.
– These equations involving the nuclei of the reactants and products are called nuclear equations.
– The nuclear reactions occur by redistribution of protons and neutrons present in the reactants to form the products.
– Thus in the Nuclear equation:
(1) the total number of protons and neutrons in the reactants and products is the same.
(2) the sum of the mass numbers and atomic numbers on the two sides of the equation must be equal.
– If the mass numbers and atomic numbers of all but one of the atoms or particles in a nuclear reaction are known, the unknown particle can be identified.
How to write Nuclear Equations?
(1) Write the symbols of the nuclei and particles including the mass numbers (superscripts) and atomic numbers (subscripts) on the left (reactants) and right (products) of the arrow.
(2) Balance the equation so that the sum of the mass numbers and atomic numbers of the particles (including the unknown) on the two sides of the equation are equal.
– Thus find the atomic number and mass number of the unknown atom, if any.
(3) Then look at the periodic table and identify the unknown atom whose atomic number is disclosed by the balanced equation.
Examples of Nuclear equations
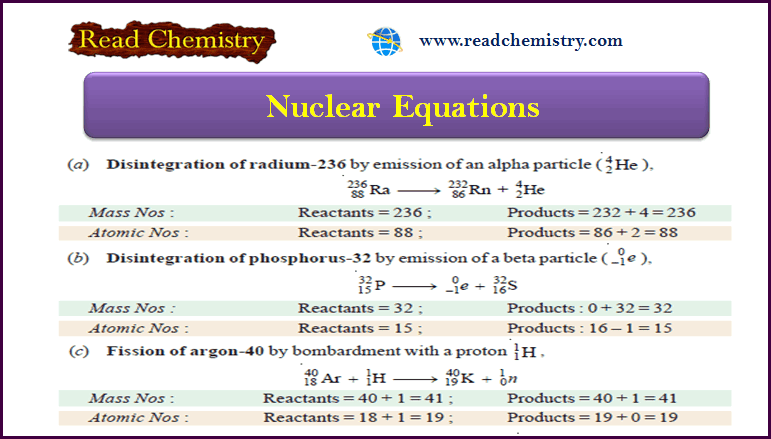
(a) Disintegration of radium-236 by emission of an alpha particle (4He2).
(b) Disintegration of phosphorus-32 by emission of a beta particle (0e–1).
(c) Fission of argon-40 by bombardment with a proton 1H1 .
(d) Fission of uranium-235 by absorption of a neutron (1n0).
(e) Fusion of lithium-7 and proton, 1H1.
Solved Problem
Problem (1)
Write the nuclear equation for the change that occurs in radium-226 when it emits an alpha particle.
Solution:
Step 1: Write the symbol of the parent atom with its mass number and atomic number (from the periodic table) on the left-hand side of the equation.
Step2: Write the symbol for the alpha particle on the right-hand side of the equation.
Step 3: Complete the equation by writing the symbol of an isotope that has an atomic number 88 – 2 = 86 and mass number 226 – 4 = 222. As shown by the periodic table, the isotope with atomic number 86 is radon, Rn. Thus,
Step 4: Check that the mass numbers and atomic numbers on the two sides of the equation are balanced.
Problem (2)
Cobalt–60 decays by emission of a beta particle. Predict the atomic number, mass number, and name of the isotope formed.
Solution:
Step 1: Write the symbol of the cobalt with the mass number and atomic number (from the periodic table) on the left-hand side of the equation.
Step2: If the unknown product isotope is X with mass number M and atomic number A, the nuclear equation may be written as:
Step 3: Since the sum of mass numbers and atomic numbers are equal on the two sides of the equation:
Step 4: Consult the periodic table and find the element whose atomic number is 28. This is nickel, Ni.
– Therefore, the complete equation for the decay of cobalt-60 is:
Problem (3)
Complete the nuclear equation
Solution:
– Let the unknown atom be X with mass number M and atomic number A.
– We can write the above nuclear equation as:
– But the sum of mass numbers on the two sides of the equation is equal. Thus
– Find the isotope of atomic number 94 from the periodic table. It is plutonium, Pu.
– Thus the completed nuclear equation is:
Reference: Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolor edition.