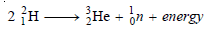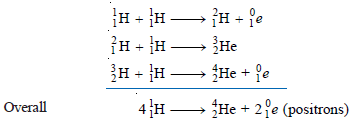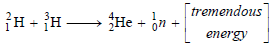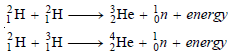Nuclear Fusion: Definition, Occurrence, Examples, Applications
– In this subject, we will discuss the Nuclear Fusion Process ( Definition, Occurrence, Examples, Applications)
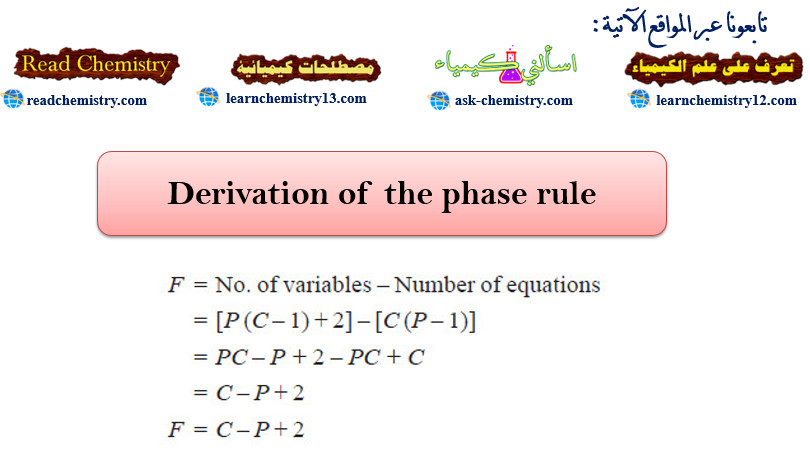
Nuclear Fusion Process
– The Nuclear Fusion Process is the opposite of nuclear fission.
– Nuclear fusion may be defined as the process in which two lightweight nuclei combine or fuse to form a single heavier nucleus.
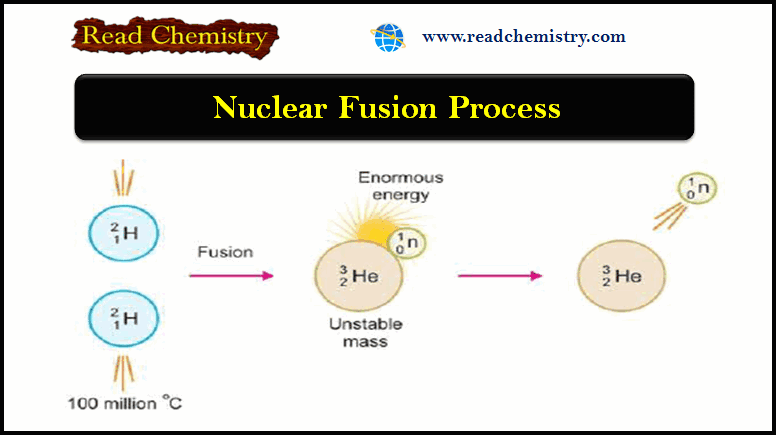
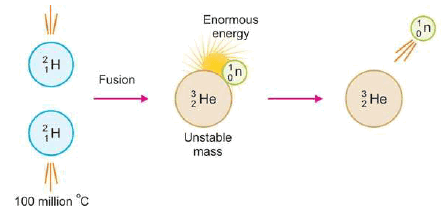
– The following figure shows an Illustration of the fusion of two deuterium (2H1) nuclei to form a single nucleus of helium (3He2) with the release of a neutron and enormous energy.
– For example, two nuclei of deuterium (2H1) undergo nuclear fusion to yield a heavier nucleus of helium-3.
– This fusion reaction takes place at a temperature of about 100 million °C.
– The above fusion reaction may be stated in the form of an equation as:
– Since fusion reactions occur at extremely high temperatures, these are also called thermonuclear reactions.
– In a fusion reaction, the mass of the reacting nuclei is greater than that of the nucleus formed.
– The differential mass is manifested in the great amount of energy released in the reaction.
– For example:
– The total mass of the reactants is 4.02277 amu which is 0.02017 amu greater than the mass of the product.
– The mass that is lost is covered by a lot of energy.
– A pair of reacting nuclei induces the fusion of another pair of nuclei. In this way, fantastic amounts of energy are generated.
– This is the basis of the H-bomb or Hydrogen bomb.
How does Nuclear Fusion occur?
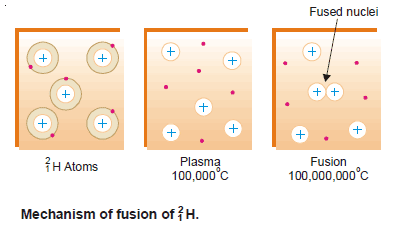
– Let us explain the mechanism of fusion by taking the example of the fusion of deuterium (2H1) cited above.
(1) At extremely high temperatures, 100 million °C or more, atoms do not exist as such.
(2) At these temperatures, deuterium atoms are completely stripped of orbital electrons.
(3) Thus results a system containing positive nuclei and electrons, called plasma.
– In this state, the high kinetic energy of the nuclei can overcome electrostatic repulsions between them.
(4) The nuclei collide with such great force that they merge or fuse to form larger nuclei.
Solar Energy
– Solar Energy is The energy released by the sun results from a series of nuclear fusion reactions.
– The overall reaction consists of the fusion of four hydrogen nuclei (protons) to form a helium nucleus.
– One mechanism suggested for the process is:
– The fusion reactions in the sun take place at exceedingly high temperatures-greater than 40 million °C.
– Every second the sun loses 4.3 × 109 kg (4, 20,000 tons) of mass by the fusion reactions.
– This mass is converted to energy.
– But the total mass of the sun is so great that its loss of mass is imperceptible.
– It is hoped that the sun will continue to pour energy on the earth for billions of years
Hydrogen Bomb or H-Bomb
– This deadly device makes use of the nuclear fusion of the isotopes of hydrogen.
– It consists of a small plutonium fission bomb with a container of isotopes of hydrogen.
– While the exact reaction used is a strictly guarded military secret, a fusion reaction between H-2 and H-3 may be the possible source of the tremendous energy released
– The fusion bomb produces the high temperature required for nuclear fusion and triggers the H-bomb.
– The explosion of such a bomb is much more powerful than that of a fission bomb or atomic bomb.
– Fortunately, the H-bombs have been tested and are not used in actual warfare.
– If they are ever used, it may mean the end of civilization on earth.
Fusion as a Source of Energy In the 21st Century
– Almost likely that the world’s energy source in the twenty-first century will be a fusion reactor.
– As indicated by the trends of research, it will be based on the reactions:
– A fusion reactor thus developed will be any time superior to a fission reactor for generating electricity.
(1) The fusion fuel, deuterium (2H1), can be obtained in abundance from heavy water present in seawater.
– The supplies of U-235 needed for a fission reactor are limited.
(2) A fusion reaction produces considerably greater energy per gram of fuel than a fission reaction.
(3) The products of fusion (3He2, 4He2) are not radioactive.
– Thus, there will be no problem with waste disposal.
– So far, it has not been possible to set up a fusion reactor.
– The chief difficulty is that the reactant nuclei must be heated to very high temperatures.
– A mixture of deuterium and tritium nuclei, for example, requires 30 million °C before they can fuse.
– So far no substance is known that can make a container that could withstand such high temperatures.
– However, scientists are making efforts to effect fusion at a lower temperature with the help of laser beams.
Reference: Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolor edition.