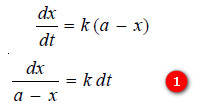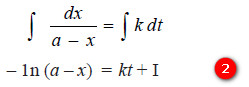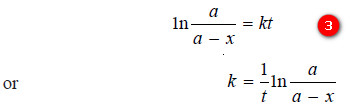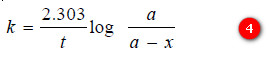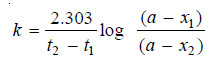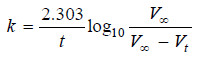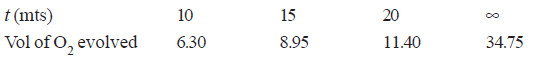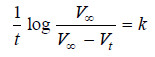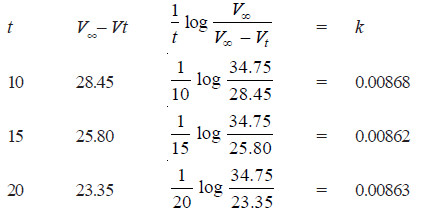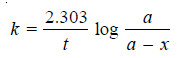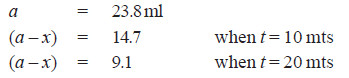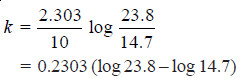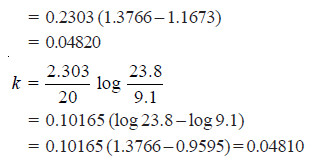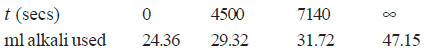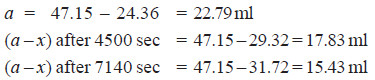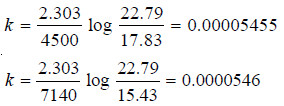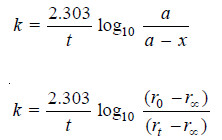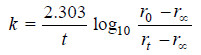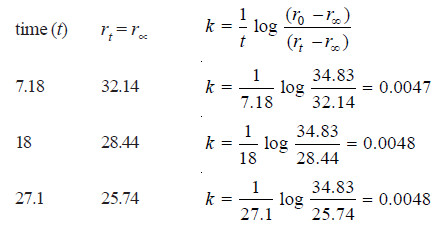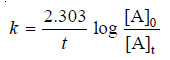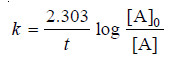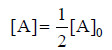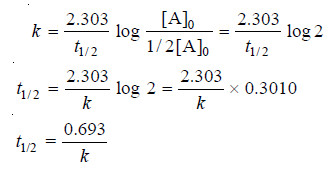First Order Reaction -Examples and Solved problems
First order reaction
– Let us consider a first order reaction:
A → products
– Suppose that at the beginning of the reaction (t = 0), the concentration of A is a moles liter–1.
– If after time t, x moles of A have changed, the concentration of A is a – x.
– We know that for a first-order reaction, the rate of reaction, dx/dt, is directly proportional to the concentration of the reactant. Thus,
– Integration of the expression (1) gives:
– where (I) is the constant of integration.
– The constant k may be evaluated by putting t = 0 and x = 0. Thus,
I = – ln a
– Substituting for (I) in equation (2):
– Changing into common logarithms:
– The value of k can be found by substituting the values of a and (a – x) determined experimentally at time interval t during the course of the reaction.
– Sometimes the integrated rate law in the following form is also used:
where x1 and x2 are the amounts decomposed at time intervals t1 and t2 respectively from the start.
Examples of First order reactions
Some common reactions which follow first order kinetics are listed below :
(1) Decomposition of N2O5 in CCl4 solution
– Nitrogen pentoxide in carbon tetrachloride solution decomposes to form oxygen gas,
N2O5 → 2NO2 + 1/2 O2
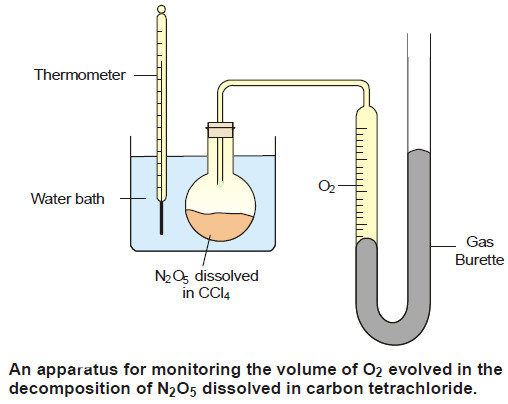
– The reaction is carried out in an apparatus shown in the following Figure:
– The progress of the reaction is monitored by measuring the volume of oxygen evolved from time to time.
– If Vt be the volume of O2 at any time t and V∞ the final volume of oxygen when the reaction is completed, the V∞ is a measure of the initial concentration of N2O5 and (V∞ – Vt) is a measure of undecomposed N2O5 (a – x) remaining at time t. Thus,
– On substituting values of V∞ , (V∞ – Vt) at different time intervals, t, the value of k is found to be constant. Thus it is a reaction of the first order.
Solved Problem (1): From the following data for the decomposition of N2O5 in CCl4 solution at 48°C, show that the reaction is of the first order
Solution:
– For a first order reaction, the integrated rate equation is:
– In this example, V∞ = 34.75
Since the value of k is fairly constant, it is a first order reaction.
(2) Decomposition of H2O2 in aqueous solution
– The decomposition of H2O2 in the presence of Pt as catalyst is a first order reaction.
– The progress of the reaction is followed by titrating equal volumes of the reaction mixture against standard KMnO4 solution at different time intervals.
Solved Problem (2): A solution of H2O2 when titrated against KMnO4 solution at different time intervals gave the following results :
Show that the decomposition of H2O2 is a first order reaction.
Solution:
– The integrated rate equation for first order reaction is
Since the volume of KMnO4 used in the titration is the measure of the concentration of H2O2 in the solution
– Substituting these values in the rate equation above, we have;
– Since the value of k is almost constant, the decomposition of H2O2 is a first order reaction.
(3) Hydrolysis of an Ester
– The hydrolysis of ethyl acetate or methyl acetate in the presence of a mineral acid as catalyst, is a first order reaction.
CH3COOC2H5 + H2O → CH3COOH + C2H5OH
– For studying the kinetics of the reaction, a known volume of ethyl acetate is mixed with a relatively large quantity of acid solution, say N/2 HCl.
– At various intervals of time, a known volume of the reaction mixture is titrated against a standard alkali solution.
– Hydrolysis of the ester produces acetic acid.
– Therefore as the reaction proceeds, the volume of alkali required for titration goes on increasing.
Solved Problem (3): The following data was obtained on hydrolysis of methyl acetate at 25°C in 0.35N hydrochloric acid. Establish that it is a first order reaction.
Solution:
– For a first order reaction:
– At any time, the volume of alkali used is needed for the acid present as catalyst and the acid produced by hydrolysis.
– The volume of alkali used for total change from t0 to t∝ gives the initial concentration of ester. Thus,
– Substituting values in the rate equation above, we have:
Since the values of k in the two experiments are fairly constant, the reaction is of the first order.
(4) Inversion of Cane sugar (sucrose).
– The inversion of cane sugar or sucrose catalyzed with dil HCl to give D-glucose and D-fructose follows the first order kinetics
C12H22O11 +H2O ⎯⎯→ C6H12O6 + C6H12O6
– The progress of the reaction is followed by noting the optical rotation of the reaction mixture with the help of a polarimeter at different time intervals.
– The optical rotation goes on changing since D-glucose rotates the plane of polarised light to the right and D-fructose to the left.
– The change in rotation is proportional to the amount of sugar decomposed.
– Let the final rotation be r∞, the initial rotation t0 while the rotation at any time t is rt
The initial concentration, a is ∞ ( t0 – r∝).
The concentration at time t, (a – x) is ∝ (rt – r∝)
– Substituting in the first order rate equation,
– If the experimental values of t ( t0 – r∝) and (rt – r∝) are substituted in the above equation, a constant value of k is obtained.
Solved Problem (4): The optical rotation of sucrose in 0.9N HCl at various time intervals is given in the table below.
Show that inversion of sucrose is a first order reaction.
Solution:
– The available data is substituted in the first order rate equation for different time intervals
r0 – r∝ = 24.09 – (– 10.74) = 34.83 for all time intervals. Thus, the value of rate constant can be found.
Since the value of k comes out to be constant, the inversion of sucrose is a first order reaction.
Units of First order Rate constant
The rate constant of a first order reaction is given by:
– Thus the rate constant for the first order reaction is independent of the concentration.
– It has the unit time–1
Calculation of Half-life of a First order Reaction
– Reaction rates can also be expressed in terms of half-life or half-life period.
– It is defined as the time required for the concentration of a reactant to decrease to half its initial value.
– In other words, half-life is the time required for one-half of the reaction to be completed.
– It is represented by the symbol t1/2 or t0.5.
– The integrated rate equation (4) for a first order reaction can be stated as :
where [A]0 is initial concentration and [A] is concentration at any time t. Half-life, t1/2, is time when initial concentration reduces to 1/2 i.e.,
Substituting values in the integrated rate equation, we have:
It is clear from this relation that :
(1) The half-life for a first order reaction is independent of the initial concentration.
(2) it is inversely proportional to k, the rate-constant.