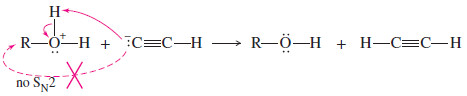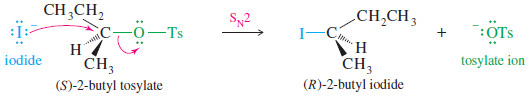Alcohols as Nucleophiles and Electrophiles
Alcohols as Nucleophiles and Electrophiles; Formation of Tosylates
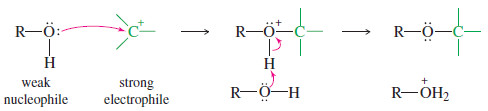
– One reason alcohols are such versatile chemical intermediates is that they react as both nucleophiles and electrophiles.
– The following scheme shows an alcohol reacting as a weak nucleophile, bonding to a strong electrophile (in this case, a carbocation).
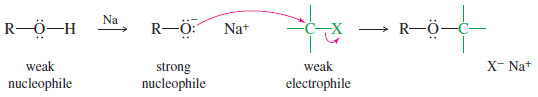
– An alcohol is easily converted to a strong nucleophile by forming its alkoxide ion.
– The alkoxide ion can attack a weaker electrophile, such as an alkyl halide.
– The O-H bond is broken when alcohols react as nucleophiles, both when an alcohol reacts as a weak nucleophile, or when an alcohol is converted to its alkoxide that then reacts as a strong nucleophile.
– In contrast, when an alcohol reacts as an electrophile, the C-O bond is broken.
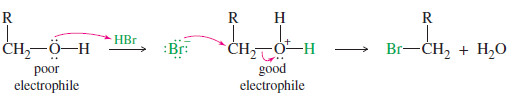
– An alcohol is a weak electrophile because the hydroxyl group is a poor leaving group.
– The hydroxyl group becomes a good leaving group (H2O) when it is protonated.
– For example, HBr reacts with a primary alcohol by an SN2 attack of bromide on the protonated alcohol. Note that the C-O bond is broken in this reaction.
– The disadvantage of using a protonated alcohol is that a strongly acidic solution is required to protonate the alcohol.
– Although halide ions are stable in acid, few other good nucleophiles are stable in strongly acidic solutions.
– Most strong nucleophiles are also basic and will abstract a proton in acid.
– Once protonated, the reagent is no longer nucleophilic.
– For example, an acetylide ion would instantly become protonated if it were added to a protonated alcohol.
– How can we convert an alcohol to an electrophile that is compatible with basic nucleophiles?
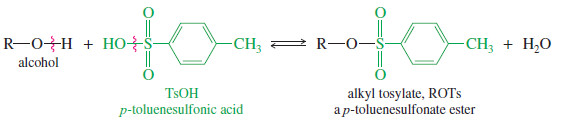
– We can convert it to an alkyl halide, or we can simply make its tosylate ester.
– A tosylate ester (symbolized ROTs) is the product of condensation of an alcohol with p- toluenesulfonic acid (symbolized TsOH).
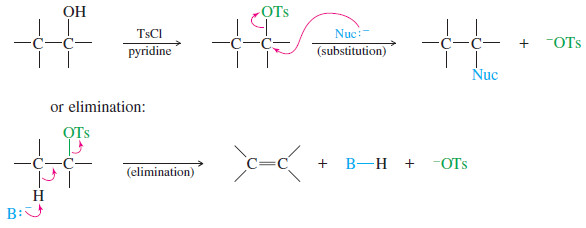
– The tosylate group is an excellent leaving group, and alkyl tosylates undergo substitution and elimination much like alkyl halides.
– In many cases, a tosylate is more reactive than the equivalent alkyl halide
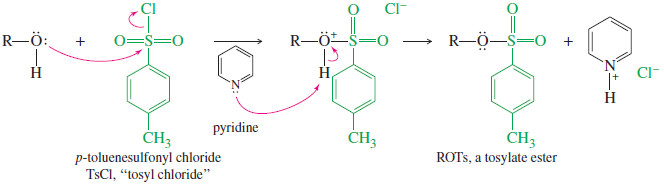
– Tosylates are made from alcohols using tosyl chloride (TsCl) in pyridine, as shown next.
– This reaction gives much higher yields than the reaction with TsOH itself.
– The mechanism of tosylate formation shows that C-O bond of the alcohol remains intact throughout the reaction, and the alcohol retains its stereochemical configuration.
– Pyridine serves as an organic base to remove the HCl formed in the reaction, preventing it from protonating the alcohol and causing side reactions.
– The following reaction shows the SN2displacement of tosylate ion (–OTs) from (S)-2-butyl tosylate with inversion of configuration.
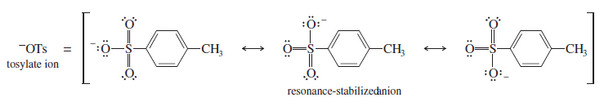
– The tosylate ion is a particularly stable anion, with its negative charge delocalized over three oxygen atoms.
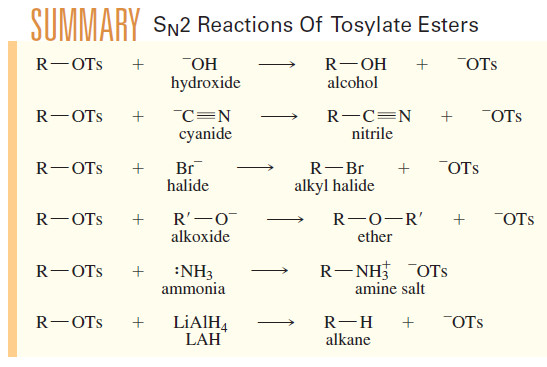
– Like halides, the tosylate leaving group is displaced by a wide variety of nucleophiles.
– The SN2 mechanism (strong nucleophile) is more commonly used in synthetic preparations than the SN1 .
– The following reactions show the generality of SN2 displacements of tosylates.
– In each case, R must be an unhindered primary or secondary alkyl group if substitution is to predominate over elimination.