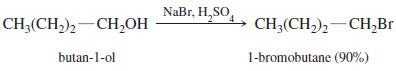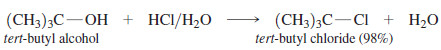Reactions of Alcohols with Hydrohalic Acids
– In this topic the Reactions of Alcohols with Hydrohalic Acids such as HBr , HCl are discussed
Reactions of Alcohols with Hydrohalic Acids
– Tosylation of an alcohol, followed by displacement of the tosylate by a halide ion, converts an alcohol to an alkyl halide.
– This is not the most common method for converting alcohols to alkyl halides, however, because simple, one-step reactions are available.
– A common method is to treat the alcohol with a hydrohalic acid, either HBr, HCl, or HI.
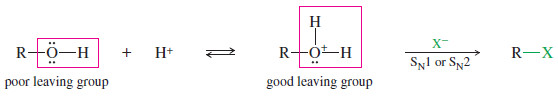
– In acidic solution, an alcohol is in equilibrium with its protonated form.
– Protonation converts the hydroxyl group from a poor leaving group (-OH) to a good leaving group(H2O).
– Once the alcohol is protonated, all the usual substitution and elimination reactions are feasible, depending on the structure (1°, 2°, 3°) of the alcohol.
– Most good nucleophiles are basic, becoming protonated and losing their nucleophilicity in acidic solutions.
– Halide ions are exceptions, however. Halides are anions of strong acids, so they are weak bases.
– Solutions of HBr, HCl, or HI contain nucleophilic Br–, Cl–, or I– ions. These acids are commonly used to convert alcohols to the corresponding alkyl halides.
Reactions of Alcohols with Hydrobromic Acid
R-OH + HBr/H2O → R-Br
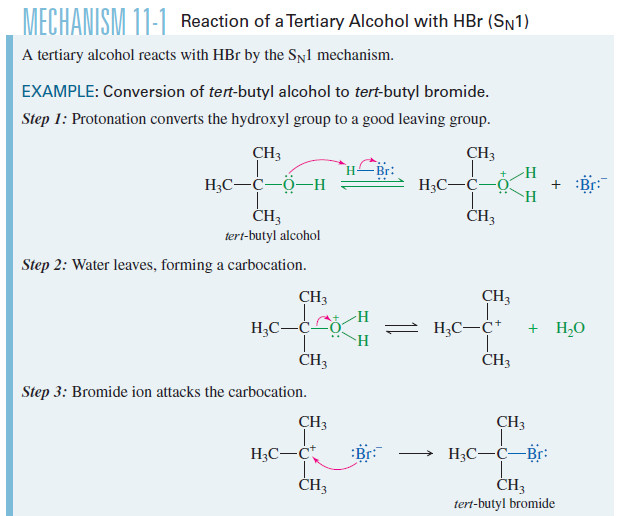
– Concentrated hydrobromic acid rapidly converts tert-butyl alcohol to tert-butyl bromide.
– The strong acid protonates the hydroxyl group, converting it to a good leaving group.
– The hindered tertiary carbon atom cannot undergo SN2 displacement, but it can ionize to a tertiary carbocation.
– Attack by bromide gives the alkyl bromide.
– The mechanism is similar to other SN1 mechanisms we have studied, except that water serves as the leaving group from the protonated alcohol.
– Many other alcohols react with HBr, with the reaction mechanism depending on the structure of the alcohol.
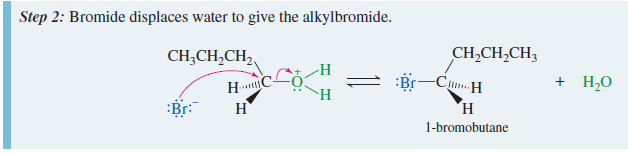
– For example, butan-1-ol reacts with sodium bromide in concentrated sulfuric acid to give 1-bromobutane by an SN2 displacement.
– The sodium bromide/sulfuric acid reagent generates HBr in the solution.
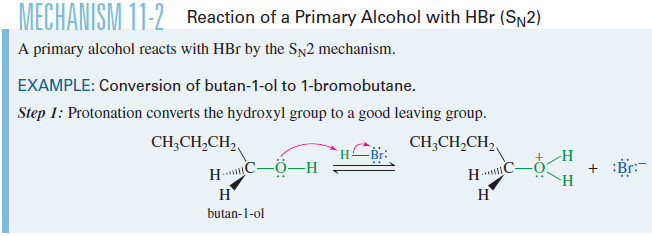
– Protonation converts the hydroxyl group to a good leaving group, but ionization to a primary carbocation is unfavorable.
– The protonated primary alcohol is well suited for the SN2 displacement, however.
– Back-side attack by bromide ion gives 1-bromobutane
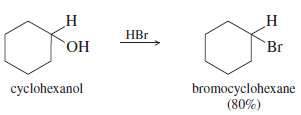
– Secondary alcohols also react with HBr to form alkyl bromides, usually by the mechanism.
– For example, cyclohexanol is converted to bromocyclohexane using HBr as the reagent.
Reactions of Alcohols with Hydrochloric Acid
– Hydrochloric acid (HCl) reacts with alcohols in much the same way that hydrobromic acid does.
– For example, concentrated aqueous HCl reacts with tert-butyl alcohol to give tert-butyl chloride.
– Chloride ion is a weaker nucleophile than bromide ion because it is smaller and less polarizable.
– An additional Lewis acid, such as zinc chloride ZnCl2 is sometimes necessary to promote the reaction of HCl with primary and secondary alcohols.
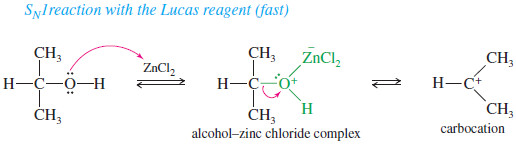
– Zinc chloride coordinates with the oxygen of the alcohol in the same way a proton does— except that zinc chloride coordinates more strongly.
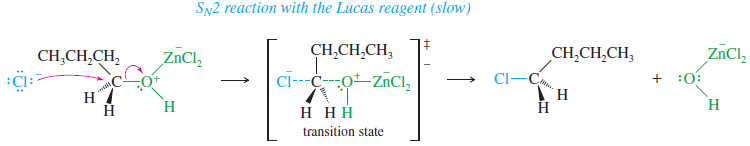
– The reagent composed of HCl and ZnCl2 is called the Lucas reagent.
– Secondary and tertiary alcohols react with the Lucas reagent by the SN1 mechanism.
SN1 reaction with the Lucas reagent (fast)
– When a primary alcohol reacts with the Lucas reagent, ionization is not possible— the primary carbocation is too unstable.
– Primary substrates react by an SN2 mechanism, which is slower than the SN1 reaction of secondary and tertiary substrates.
– For example, when butan-1-ol reacts with the Lucas reagent, the chloride ion attacks the complex from the back, displacing the leaving group.
SN2 reaction with the Lucas reagent (slow)
The Lucas Test
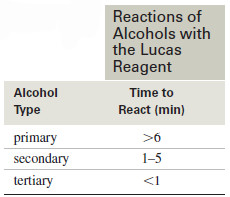
– The Lucas reagent reacts with primary, secondary, and tertiary alcohols at predictable rates, and these rates can distinguish among the three types of alcohols.
– When the reagent is first added to the alcohol, the mixture forms a single homogeneous phase: The concentrated HCl solution is very polar, and the polar alcohol–zinc chloride complex dissolves.
– Once the alcohol has reacted to form the alkyl halide, the relatively nonpolar halide separates into a second phase. (R-OH dissolves, but R-Cl does not.)
– The Lucas test involves adding the Lucas reagent to an unknown alcohol and watching for the second phase to separate (see Table).
– Tertiary alcohols react and show a second phase almost instantly because they form relatively stable tertiary carbocations.
– Secondary alcohols react in about 1 to 5 minutes because their secondary carbocations are less stable than tertiary ones. Primary alcohols react very slowly.
– Since the activated primary alcohol cannot form a carbocation, it simply remains in solution until it is attacked by the chloride ion.
– With a primary alcohol, the reaction may take from 10 minutes to several days.
Limitations on the Use of Hydrohalic Acids with Alcohols
– The reactions of alcohol with hydrohalic acids do not always give good yields of the expected alkyl halides.
– Four principal limitations restrict the generality of this technique.
(1) Poor yields of alkyl chlorides from primary and secondary alcohols
– Primary and secondary alcohols react with HCl much more slowly than tertiary alcohols, even with zinc chloride added.
– Under these conditions, side reactions may prevent good yields of the alkyl halides.
(2) Eliminations
– Heating an alcohol in a concentrated acid such as HCl or HBr often leads to elimination.
– Once the hydroxyl group of the alcohol has been protonated and converted to a good leaving group, it becomes a candidate for both substitution and elimination.
(3) Rearrangements
– Carbocation intermediates are always prone to rearrangements.
– We have seen that hydrogen atoms and alkyl groups can migrate from one carbon atom to another to form a more stable carbocation.
– This rearrangement may occur as the leaving group leaves, or it may occur once the cation has formed.
(4) Limited ability to make alkyl iodides
– Many alcohols do not react with HI to give acceptable yields of alkyl iodides.
– Alkyl iodides are valuable intermediates, however, because iodides are the most reactive of the alkyl halides.
– We will discuss another technique for making alkyl iodides in the next section.
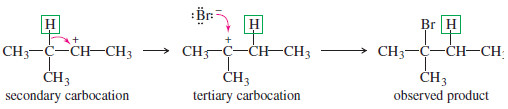
Solved problem
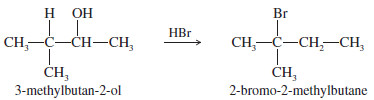
When 3-methylbutan-2-ol is treated with concentrated HBr, the major product is 2-bromo-2-methylbutane. Propose a mechanism for the formation of this product.
Solution:
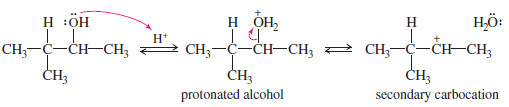
– The alcohol is protonated by the strong acid.
– This protonated secondary alcohol loses water to form a secondary carbocation.
– A hydride shift transforms the secondary carbocation into a more stable tertiary cation.
– Attack by bromide leads to the observed product.
– Although rearrangements are usually seen as annoying side reactions, a clever chemist can use a rearrangement to accomplish a synthetic goal.