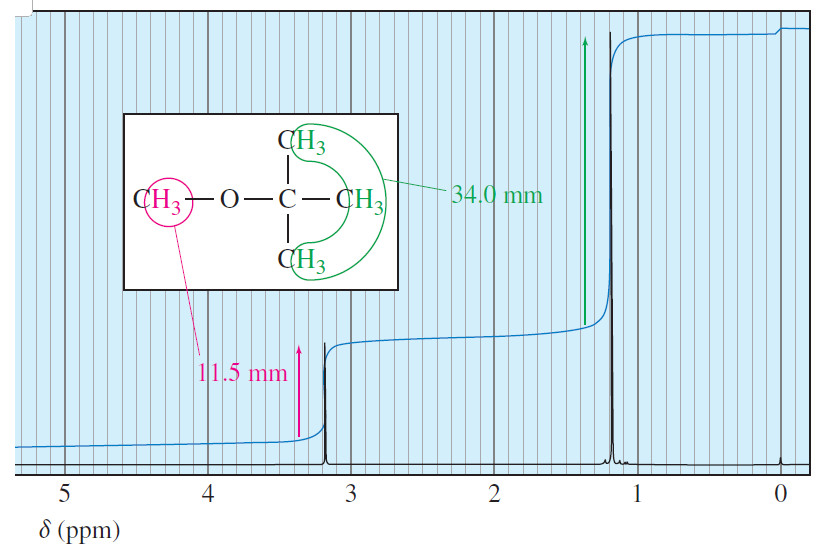
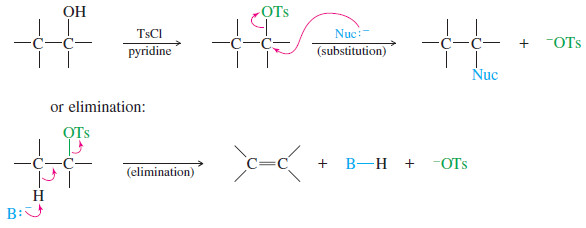
Comparison of E1 and E2 reactions
Comparison of E1 and E2 Elimination Mechanisms
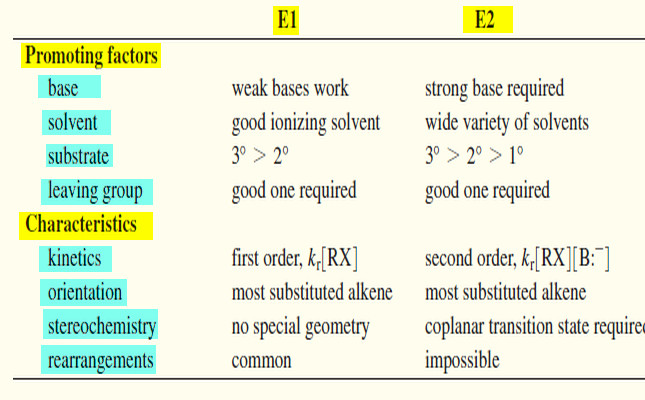
– Let’s summarize the major points to remember about the E1 and E2 reactions, focusing on the factors that help us predict which of these mechanisms will operate under a given set of experimental conditions. Then we will organize these factors into a short table.
(1) Effect of the Base on E1 and E2 reactions
– The nature of the base is the single most important factor in determining whether an elimination will go by the E1 or E2 mechanism.
– If a strong base is present, the rate of the bimolecular reaction will be greater than the rate of ionization, and the E2 reaction will predominate (perhaps accompanied by the SN2).
– If no strong base is present, then a good solvent makes a unimolecular ionization likely.
– Subsequent loss of a proton to a weak base (such as the solvent) leads to elimination.
– Under these conditions, the E1 reaction usually predominates, usually accompanied by the SN1
E1: Base strength is unimportant (usually weak).
E2: Requires strong bases.
(2) Effect of the Solvent on E1 and E2 reactions
– The slow step of the E1 reaction is the formation of two ions.
– Like the SN1, the E1 reaction critically depends on polar ionizing solvents such as water and the alcohols.
– In the E2 reaction, the transition state spreads out the negative charge of the base over the entire molecule.
– There is no more need for solvation in the E2 transition state than in the reactants.
– The E2 is therefore less sensitive to the solvent; in fact, some reagents are stronger bases in less polar solvents.
E1: Requires a good ionizing solvent.
E2: Solvent polarity is not so important.
(3) Effect of the Substrate on E1 and E2 reactions
– For both the E1 and the E2 reactions, the order of reactivity is
E1, E2: (3° > 2° >1° ( 1° usually will not go E1)
– In the E1 reaction, the rate-limiting step is formation of a carbocation, and the reactivity order reflects the stability of carbocations.
– In the E2 reaction, the more substituted halides generally form more substituted, more stable alkenes.
(4) Kinetics of E1 and E2 reactions
– The rate of the E1 reaction is proportional to the concentration of the alkyl halide [RX] but not to the concentration of the base. It follows a first-order rate equation.
– The rate of the E2 reaction is proportional to the concentrations of both the alkyl halide [RX] and the base [B:–] . It follows a second-order rate equation.
E1 rate = kr[RX]
E2 rate = kr[RX][B:–]
(5) Orientation of Elimination
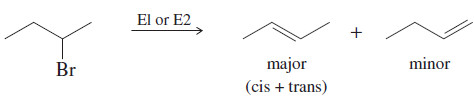
– In most E1 and E2 eliminations with two or more possible products, the product with the most substituted double bond (the most stable product) predominates.
– This principle is called Zaitsev’s rule, and the most highly substituted product is called the Zaitsev product.
E1, E2: Usually Zaitsev orientation.
(6) Stereochemistry of E1 and E2 reactions
– The E1 reaction begins with an ionization to give a flat carbocation.
– No particular geometry is required for ionization
– The E2 reaction takes place through a concerted mechanism that requires a coplanar arrangement of the bonds to the atoms being eliminated.
– The transition state is usually anti-coplanar, although it may be syn-coplanar in rigid systems.
E1: No particular geometry required for the slow step.
E2: Coplanar arrangement (usually anti) required for the transition state.
(7) Rearrangements
– The E1 reaction involves a carbocation intermediate.
– This intermediate can rearrange, usually by the shift of a hydride or an alkyl group, to give a more stable carbocation.
– The E2 reaction takes place in one step with no intermediates.
– No rearrangement is possible in the E2 reaction.
E1: Rearrangements are common.
E2: No rearrangements