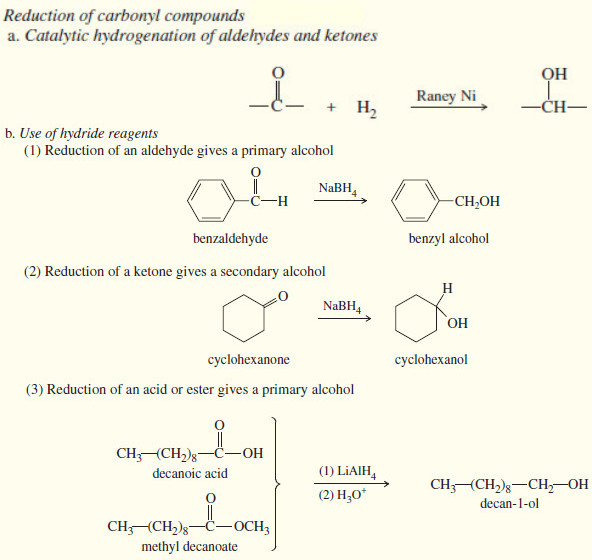
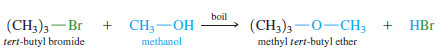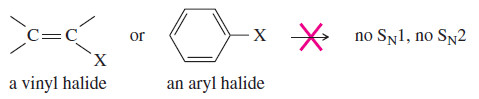SN1 Reaction – Nucleophilic Substitution reaction
SN1 Reaction: First-Order Nucleophilic Substitution
– When tert-butyl bromide is placed in boiling methanol, methyl tert butyl ether can be isolated from the reaction mixture.
– Because this reaction takes place with the solvent acting as the nucleophile, it is called a solvolysis (solvo for “solvent,” plus lysis, meaning “cleavage”)
– This solvolysis is a substitution because methoxide has replaced bromide on the tertbutyl group.
– It does not go through the SN2 mechanism, however. The SN2 requires a strong nucleophile and a substrate that is not too hindered.
– Methanol is a weak nucleophile, and tert-butyl bromide is a hindered tertiary halide—a poor SN2 substrate.
– If this substitution cannot go by the SN2 mechanism, what kind of mechanism might be involved? An important clue is kinetic: Its rate does not depend on the concentration of methanol, the nucleophile.
– The rate depends only on the concentration of the substrate, tert butyl bromide.
– This rate equation is first order overall: first order in the concentration of the alkyl halide and zeroth order in the concentration of the nucleophile.
– Because the rate does not depend on the concentration of the nucleophile, we infer that the nucleophile is not present in the transition state of the rate-limiting step.
– The nucleophile must react after the slow step.
– This type of substitution is called SN1 an reaction, for Substitution, Nucleophilic, unimolecular.
– The term unimolecular means there is only one molecule involved in the transition state of the rate-limiting step.
– The mechanism of the SN1 reaction of tertbutyl bromide with methanol is shown here.
– Ionization of the alkyl halide (first step) is the rate-limiting step.
Mechanism of The SN1 Reaction
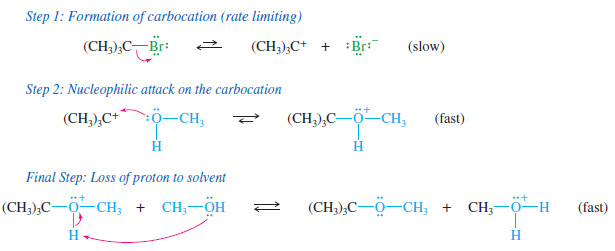
– The SN1 mechanism is a multistep process.
– The first step is a slow ionization to form a carbocation.
– The second step is a fast attack on the carbocation by a nucleophile.
– The carbocation is a strong electrophile; it reacts very fast with nucleophiles, including weak nucleophiles.
– The nucleophile in SN1 reactions is usually weak, because a strong nucleophile would be more likely to attack the substrate and force some kind of second-order reaction.
– If the nucleophile is an uncharged molecule like water or an alcohol, the positively charged product must lose a proton to give the final uncharged product.
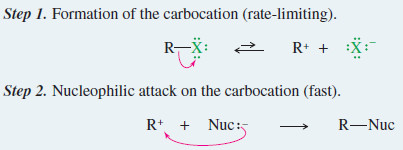
– The general mechanism for the SN1 reaction is summarized in the following Mechanism.
– The SN1 reaction involves a two-step mechanism.
– A slow ionization gives a carbocation that reacts quickly with a (usually weak) nucleophile.
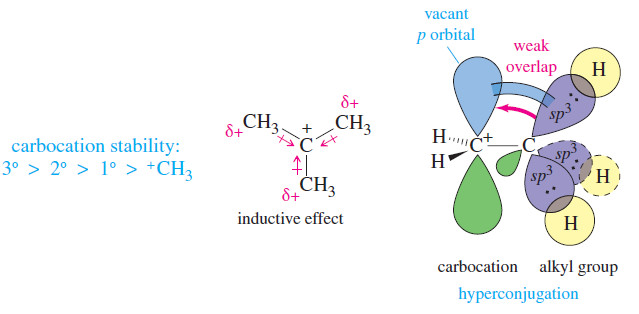
Reactivity: 3° > 2° > 1°.
Step 1. Formation of the carbocation (rate-limiting).
Step 2. Nucleophilic attack on the carbocation (fast).
If the nucleophile is water or an alcohol, a third step is needed to deprotonate the product.
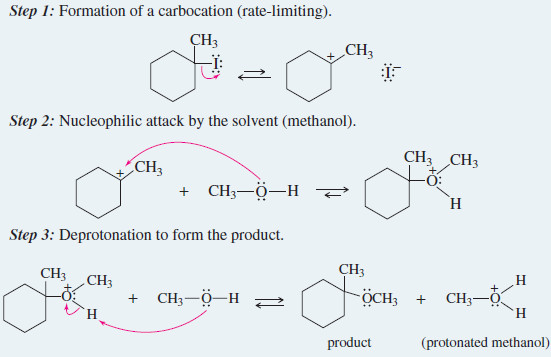
EXAMPLE: Solvolysis of 1-iodo-1-methylcyclohexane in methanol.
Step 1: Formation of a carbocation (rate-limiting).
Step. 2: Nucleophilic attack by the solvent (methanol).
Step 3: Deprotonation to form the product.
Reaction-energy diagram of The SN1 Reaction
– The reaction-energy diagram of the SN1 reaction (see Figure) shows why the rate does not depend on the strength or concentration of the nucleophile.
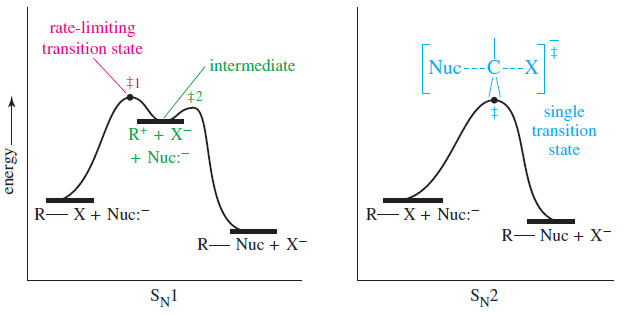
– The ionization (first step) is highly endothermic, and its large activation energy determines the overall reaction rate.
– The nucleophilic attack (second step) is strongly exothermic, with a lower-energy transition state.
– In effect, a nucleophile reacts with the carbocation almost as soon as it forms.
– The reaction-energy diagrams of the SN1 mechanism and the SN2 mechanism are compared in the preivous Figure.
– The SN1 has a true intermediate, the carbocation.
– The intermediate appears as a relative minimum (a low point) in the reaction-energy diagram.
– Reagents and conditions that favor formation of the carbocation (the slow step) accelerate the SN1 reaction; reagents and conditions that hinder its formation retard the reaction.
Substituent Effects on SN1 Reaction
– The rate-limiting step of the SN1 reaction is ionization to form a carbocation, a strongly endothermic process.
– The first transition state resembles the carbocation (Hammond postulate); consequently, rates of SN1 reactions depend strongly on carbocation stability.
– we saw that alkyl groups stabilize carbocations by donating electrons through sigma bonds (the inductive effect) and through overlap of filled orbitals with the empty p orbital of the carbocation (hyperconjugation).
– Highly substituted carbocations are therefore more stable.
Reactivity toward SN1 substitution mechanisms follows the stability of carbocations:
- This order is opposite that of the SN2 reaction.
– Alkyl groups hinder the SN2 by blocking attack of the strong nucleophile, but alkyl groups enhance the SN1 by stabilizing the carbocation intermediate.
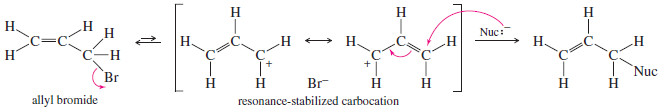
– Resonance stabilization of the carbocation can also promote the SN1 reaction.
– For example, allyl bromide is a primary halide, but it undergoes the SN1 reaction about as fast as a secondary halide.
– The carbocation formed by ionization is resonance stabilized, with the positive charge spread equally over two carbon atoms.
– Vinyl and aryl halides generally do not undergo SN1 or SN2 reactions.
-An SN1 reaction would require ionization to form a vinyl or aryl cation, either of which is less stable than most alkyl carbocations.
– An SN2 reaction would require back-side attack by the nucleophile, which is made impossible by the repulsion of the electrons in the double bond or aromatic ring.
Leaving-Group Effects on SN1 Reaction
– The leaving group is breaking its bond to carbon in the rate-limiting ionization step of the SN1 mechanism.
– A highly polarizable leaving group helps stabilize the rate-limiting transition state through partial bonding as it leaves.
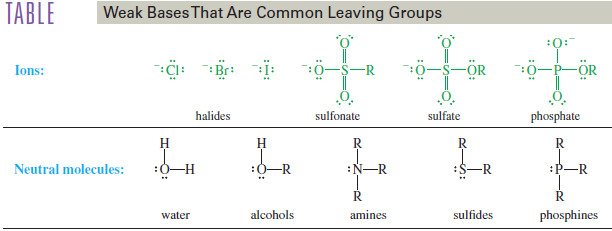
– The leaving group should be a weak base, very stable after it leaves with the pair of electrons that bonded it to carbon.
– The following figure shows the transition state of the ionization step of the SN1 reaction.
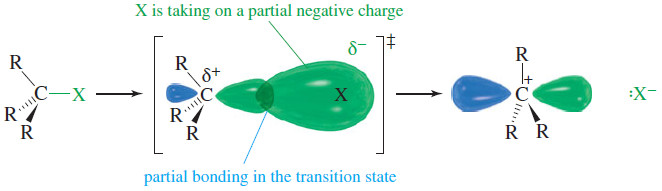
– Notice how the leaving group is taking on a negative charge while it stabilizes the new carbocation through partial bonding.
– The leaving group should be stable as it takes on this negative charge, and it should be polarizable to engage in effective partial bonding as it leaves.
– A good leaving group is just as necessary in the SN1 reaction as it is in the SN2 , and similar leaving groups are effective for either reaction.
– The following Table lists some common leaving groups for either reaction.
Solvent Effects
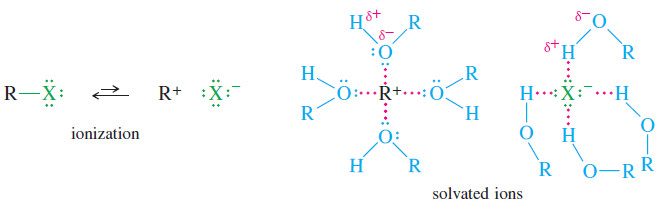
– The SN1 reaction goes much more readily in polar solvents that stabilize ions.
– The ratelimiting step forms two ions, and ionization is taking place in the transition state.
– Polar solvents solvate these ions by an interaction of the solvent’s dipole moment with the charge of the ion.
– Protic solvents such as alcohols and water are even more effective solvents because anions form hydrogen bonds with the -OH hydrogen atom, and cations complex with the nonbonding electrons of the -OH oxygen atom.
– Ionization of an alkyl halide requires formation and separation of positive and negative charges, similar to what happens when sodium chloride dissolves in water.
– Therefore, SN1 reactions require highly polar solvents that strongly solvate ions.
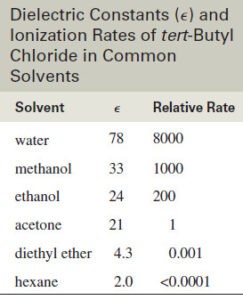
– One measure of a solvent’s ability to solvate ions is its dielectric constant a measure of the solvent’s polarity.
– The following table lists the dielectric constants of some common solvents and the relative ionization rates for tert-butyl chloride in these solvents.
– Note that ionization occurs much faster in highly polar solvents such as water and alcohols.
– Although most alkyl halides are not soluble in water, they often dissolve in highly polar mixtures of acetone and alcohols with water.