Physical and Chemical properties of Matter
– In this topic, we will discuss The Physical and Chemical properties of Matter and The Physical and Chemical Changes.
Physical and Chemical properties
– To distinguish among samples of different kinds of matter, we determine and compare their properties.
– We recognize different kinds of matter by their properties, which are broadly classified into chemical properties and physical properties.
Chemical properties
– Chemical properties are exhibited by matter as it undergoes changes in composition.
– These properties of substances are related to the kinds of chemical changes that the substances undergo.
– For instance, we have already described the combination of metallic magnesium with gaseous oxygen to form magnesium oxide, a white powder.
– A chemical property of magnesium is that it can combine with oxygen, releasing energy in the process.
– A chemical property of oxygen is that it can combine with magnesium.
Physical properties
– All substances also exhibit physical properties that can be observed in the absence of any change in composition.
– Color, density, hardness, melting point, boiling point, and electrical and thermal conductivities are physical properties.
– Some physical properties of a substance depend on the conditions, such as temperature and pressure, under which they are measured.
– For instance, water is a solid (ice) at low temperatures but is a liquid at higher temperatures.
– At still higher temperatures, it is a gas (steam). As water is converted from one state to another, its composition is constant.
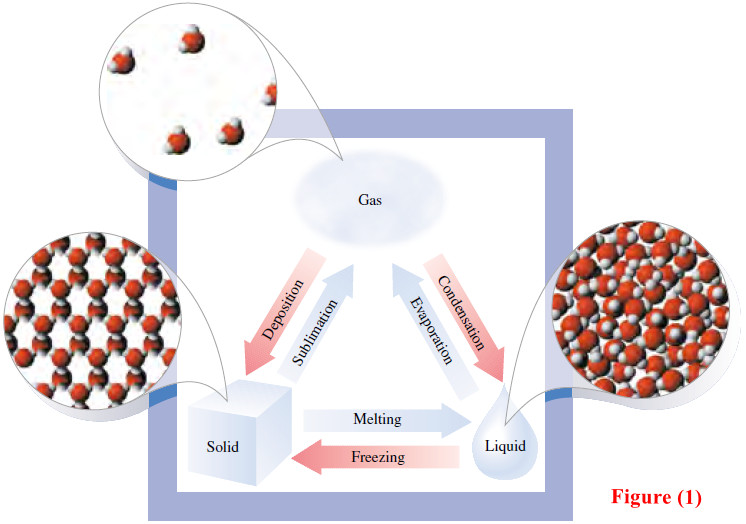
Its chemical properties change very little. On the other hand, the physical properties of ice, liquid water, and steam are different (Figure 1).
– Figure (1) shows the Physical changes that occur among the three states of matter.
– Sublimation is the conversion of a solid directly to a gas without passing through the liquid state; the reverse of that process is called deposition.
– The changes shown in blue are endothermic (absorb heat); those shown in red are exothermic (release heat). Water is a substance that is familiar to us in all three physical states.
– The molecules are close together in the solid and the liquid but far apart in the gas.
– The molecules in the solid are relatively fixed in position, but those in the liquid and gas can flow around each other.
Extensive properties and Intensive properties
– Properties of matter can be further classified according to whether or not they depend on the amount of substance present.
– The volume and the mass of a sample depend on, and are directly proportional to, the amount of matter in that sample.
– Such properties, which depend on the amount of material examined, are called extensive properties.
– By contrast, the color and the melting point of a substance are the same for a small sample and for a large one.
– Properties such as these, which are independent of the amount of material examined, are called intensive properties.
– All chemical properties are intensive properties.
– Because no two different substances have identical sets of chemical and physical properties under the same conditions, we are able to identify and distinguish among different substances.
– For instance, water is the only clear, colorless liquid that freezes at 0°C, boils at 100°C at one atmosphere of pressure, dissolves a wide variety of substances (e.g., copper(II) sulfate), and reacts violently with sodium (Figure 2).
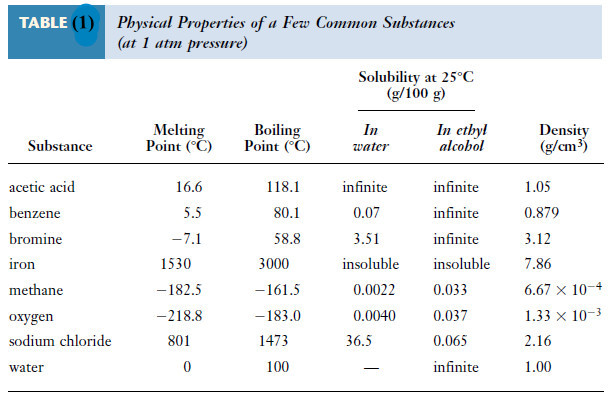
– Table (1) compares several physical properties of a few substances.
– A sample of any of these substances can be distinguished from the others by observing their properties.
Chemical and Physical Changes
– The reaction of magnesium when burns in the oxygen of the air is a chemical change.
– In any chemical change:
(1) one or more substances are used up (at least partially)
(2) one or more new substances are formed
(3) energy is absorbed or released.
– As substances undergo chemical changes, they demonstrate their chemical properties.
– A physical change, on the other hand, occurs with no change in chemical composition.
– Physical properties are usually altered significantly as matter undergoes physical changes (Figure 1).
– In addition, a physical change may suggest that a chemical change has also taken place.
– For instance, a color change, a warming, or the formation of a solid when two solutions are mixed could indicate a chemical change.
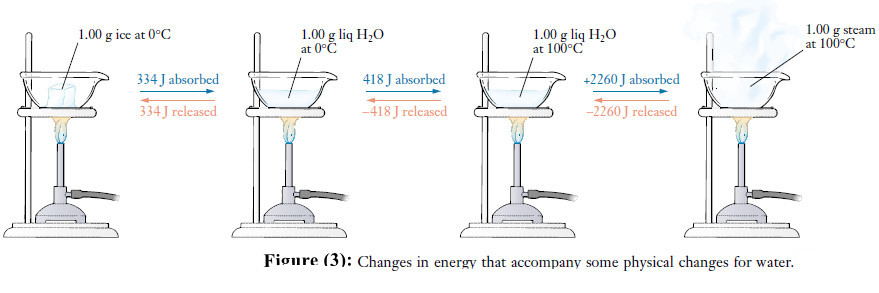
– Energy is always released or absorbed when chemical or physical changes occur.
– Energy is required to melt ice, and energy is required to boil water.
– Conversely, the condensation of steam to form liquid water always liberates energy, as does the freezing of liquid water to form ice.
– The changes in energy that accompany these physical changes for water are shown in Figure (3).
– At a pressure of one atmosphere, ice always melts at the same temperature (0°C), and pure water always boils at the same temperature (100°C).
References:
- Principles of Inorganic Chemistry / Brian W. Pfennig / 1st ed, 2015 /John Wiley & Sons, Inc/ USA.
- Inorganic Chemistry /Peter Atkins, Tina Overton, Jonathan Rourkel, Mark Weller, Fraser Armstrong, Mike Hagerman / 6th ed, 2014 /W. H. Freeman and Company/ New York, USA.
-