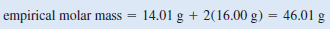General Chemistry
Experimental Determination of Empirical Formulas
Experimental Determination of Empirical Formulas
** The fact that we can determine the empirical formula of a compound if we know the percent composition enables us to identify compounds experimentally.
** The procedure is as follows.
(1) Chemical analysis tells us the number of grams of each element present in a given amount of a compound.
(2) We convert the quantities in grams to number of moles of each element.
(3) Using a certain method (we will illustrate it under) , we find the empirical formula of the compound.
** As a specific example, let us consider the compound ethanol. When ethanol is burned in an apparatus such as that shown in the following Figure.
Carbon dioxide (CO2) and water (H2O) are given off. Because neither carbon nor hydrogen was in the inlet gas, we can conclude that both carbon (C) and hydrogen (H) were present in ethanol and that oxygen (O) may also be present. (Molecular oxygen was added in the combustion process, but some of the oxygen may also have come from the original ethanol sample.)
** The masses of CO2 and of H2O produced can be determined by measuring the increase in mass of the CO2 and H2O absorbers, respectively. Suppose that in one experiment the combustion of 11.5 g of ethanol produced 22.0 g of CO2 and 13.5 g of H2O. We can calculate the mass of carbon and hydrogen in the original 11.5-g sample of ethanol as follows:
** Thus, 11.5 g of ethanol contains 6.00 g of carbon and 1.51 g of hydrogen. The remainder must be oxygen, whose mass is:
** The number of moles of each element present in 11.5 g of ethanol is:
** The formula of ethanol is therefore C0.50H1.5O0.25 (we round off the number of moles to two significant figures). Because the number of atoms must be an integer, we divide the subscripts by 0.25, the smallest subscript, and obtain for the empirical formula C2H6O.
** Now we can better understand the word (empirical) which literally means (based only on observation and measurement). The empirical formula of ethanol is determined from analysis of the compound in terms of its component elements. No knowledge of how the atoms are linked together in the compound is required.
Determination of Molecular Formulas
** The formula calculated from percent composition by mass is always the empirical formula because the subscripts in the formula are always reduced to the smallest whole numbers.
** To calculate the actual, molecular formula we must know the approximate molar mass of the compound in addition to its empirical formula.
** Knowing that the molar mass of a compound must be an integral multiple of the molar mass of its empirical formula, we can use the molar mass to find the molecular formula.
Solved problem
A sample of a compound contains 1.52 g of nitrogen (N) and 3.47 g of oxygen (O). The molar mass of this compound is between 90 g and 95 g. Determine the molecular formula and the accurate molar mass of the compound.
Strategy:
** To determine the molecular formula, we first need to determine the empirical formula. How do we convert between grams and moles? Comparing the empirical molar mass to the experimentally determined molar mass will reveal the relationship between the empirical formula and molecular formula.
Solution:
** We are given grams of N and O. Use molar mass as a conversion factor to convert grams to moles of each element. Let n represent the number of moles of each element. We write:
Thus, we arrive at the formula N0.108O0.217, which gives the identity and the ratios of atoms present. However, chemical formulas are written with whole numbers. Try to convert to whole numbers by dividing the subscripts by the smaller subscript (0.108). After rounding off, we obtain NO2 as the empirical formula.
** The molecular formula might be the same as the empirical formula or some integral multiple of it (for example, two, three, four, or more times the empirical formula). Comparing the ratio of the molar mass to the molar mass of the empirical formula will show the integral relationship between the empirical and molecular formulas. The molar mass of the empirical formula NO2 is:
Next, we determine the ratio between the molar mass and the empirical molar mass
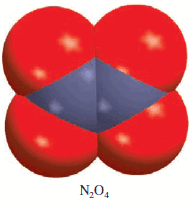
** The molar mass is twice the empirical molar mass. This means that there are two NO2units in each molecule of the compound, and the molecular formula is (NO2)2or N2O4.
** The actual molar mass of the compound is two times the empirical molar mass, that is, 2(46.01 g) or 92.02 g, which is between 90 g and 95 g.
Check:
Note that in determining the molecular formula from the empirical formula, we need only know the approximate molar mass of the compound. The reason is that the true molar mass is an integral multiple (1×, 2×, 3×, . . .) of the empirical molar mass. Therefore, the ratio (molar mass/empirical molar mass) will always be close to an integer.
Reference: General Chemistry: The Essential Concepts / Raymond Chang , Jason Overby. (sixth edition).