Photoelectric Effect and Compton Effect
– In this subject, we will discuss the Photoelectric Effect and Compton Effect
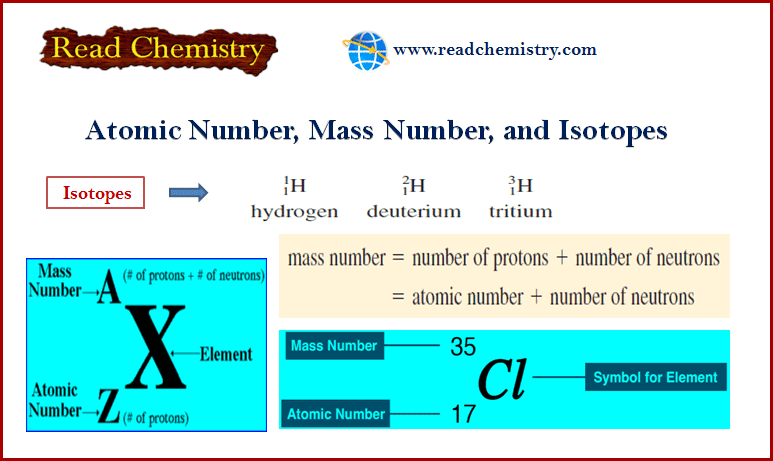
Photoelectric Effect
– When a beam of light of sufficiently high frequency is allowed to strike a metal surface in a vacuum, electrons are ejected from the metal surface.
– This phenomenon is known as the Photoelectric effect and the ejected electrons Photoelectrons.
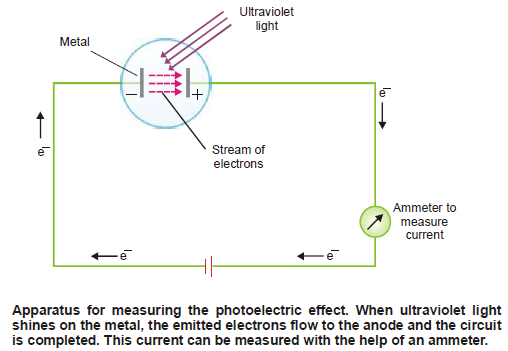
– For example, when ultraviolet light shines on Cs (or Li, Na, K, Rb) as in the apparatus shown in the following figure, the photoelectric effect occurs.
– With the help of this photoelectric apparatus, the following observations can be made:
(1) An increase in the intensity of incident light does not increase the energy of the photoelectrons. It merely increases their rate of emission.
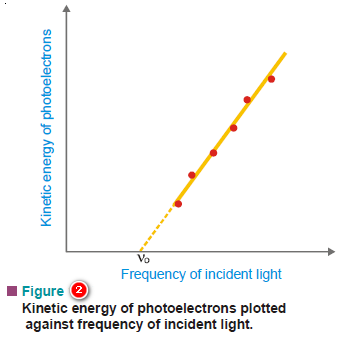
(2) The kinetic energy of the photoelectrons increases linearly with the frequency of the incident light.
– If the frequency is decreased below a certain critical value (Threshold frequency, ν0), no electrons are ejected at all.
– Classical Physics predicts that the kinetic energy of the photoelectrons should depend on the intensity of light and not on the frequency. Thus it fails to explain the above observations.
Einstein’s Explanation of Photoelectric Effect
– In 1905 Albert Einstein, who was awarded the Nobel Prize for his work on photons, interpreted the Photoelectric effect by application of the Quantum theory of light.
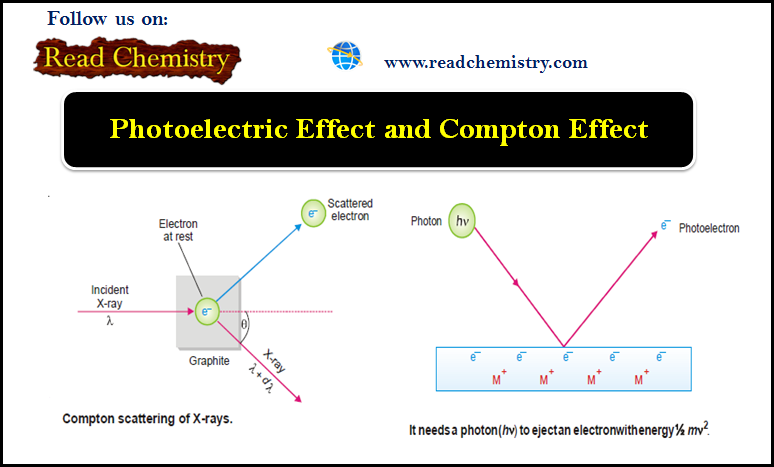
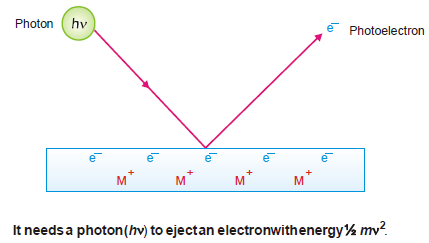
(1) A photon of incident light transmits its energy (hν) to an electron in the metal surface which escapes with kinetic energy ½ mv2.
– The greater intensity of incident light merely implies a greater number of photons each of which releases one electron.
– This increases the rate of emission of electrons, while the kinetic energy of individual photons remains unaffected.
(2) To release an electron from the metal surface, the incident photon has first to overcome the attractive force exerted by the positive ion of the metal.
– The energy of a photon (hν) is proportional to the frequency of incident light.
– The frequency which provides enough energy just to release the electron from the metal surface, will be the threshold frequency, ν0.
– For frequency less than ν0, no electrons will be emitted.
– For higher frequencies ν > ν0 a part of the energy goes to loosen the electron and remains for imparting kinetic energy to the photoelectron.
– Thus,
– Where hν is the energy of the incoming photon, hν0 is the minimum energy for an electron to escape from the metal, and ½ mv2 is the kinetic energy of the photoelectron.
– hν0 is constant for a particular solid and is designated as W, the work function.
– Rearranging equation (1):
– This is the equation for a straight line that was experimentally obtained in Fig (2).
– Its slope is equal to h, the Planck’s constant.
– The value of h thus found came out to be the same as was given by Planck himself.
Solved Problem
Problem (1): What is the minimum energy that photons must possess in order to produce photoelectric effect with platinum metal? The threshold frequency for platinum is 1.3 × 1015 sec– 1.
Solution:
– The threshold frequency (ν0) is the lowest frequency that photons may possess to produce the photoelectric effect.
– The energy corresponding to this frequency is the minimum energy (E).
– we have here:
Problem (2): Calculate the kinetic energy of an electron emitted from a surface of potassium metal (work function = 3.62 × 10–12 erg) by light of wavelength 5.5 × 10–8 cm.
Solution:
– we have here:
– Thus the electron will be emitted with kinetic energy of 3.63 × 10– 9 erg.
Compton Effect
– In 1923 A.H. Compton provided one more proof to the quantum theory or the photon theory.
– He was awarded the Nobel Prize in 1927 for his discovery of what is now called the Compton Effect.
– He demonstrated that: When X-rays of wavelength λ’ struck a sample of graphite, an electron was ejected and the X-rays scattered at an angle θ had longer wavelength λ.
Explanation of the Compton Effect
– Compton said that it was like a ball hitting a stationary ball which is pushed away while the energy of the striking ball decreases.
– Thus he argued that light radiation (X-rays) consisted of particles (photons), as a continuous wave could not have knocked out the electron.
– He visualized that a photon of incident light struck a stationary electron in graphite and hence lost some energy which resulted in the increase of wavelength.
– This process could not have occurred unless light radiation consisted of particles or photons.
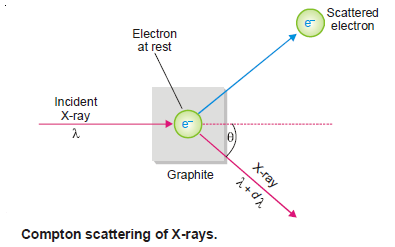
– By assuming photon-electron collisions to be perfectly elastic, Compton found that the shift in wavelength, dλ was given by the expression:
where:
- (h) is Planck’s constant,
- (m) the mass of an electron,
- (c) the velocity of light
- (θ) the angle of scattering.
– The expression shows that (dλ) is independent of the nature of the substance and wavelength of the incident radiation.
– Given the wavelength of a photon, one can calculate the momentum of the electron ejected.
Reference: Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolor edition.