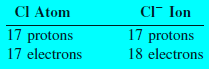Atoms, Molecules, and Ions: Definition, Types, Examples
– In this subject, we will discuss the Atoms, Molecules, and Ions: Definition, Types, Examples
Atoms
– Of all the elements, only the six noble gases in Group 8A of the periodic table (He, Ne, Ar, Kr, Xe, and Rn) exist in nature as single atoms.
– For this reason, they are called monatomic (meaning a single atom) gases.
– Most matter is composed of molecules or ions formed by atoms.
Molecules
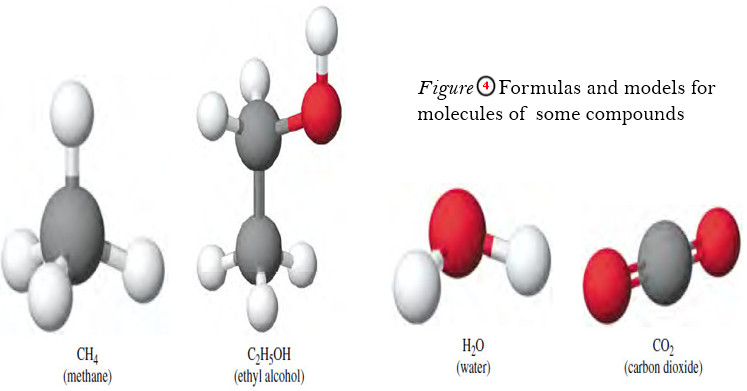
– A molecule is an aggregate of at least two atoms in a definite arrangement held together by chemical forces (also called chemical bonds).
– A molecule may contain atoms of the same element or atoms of two or more elements joined in a fixed ratio, by the law of definite proportions.
– Thus, a molecule is not necessarily a compound, which, by definition, is made up of two or more elements.
– Hydrogen gas, for example, is a pure element, but it consists of molecules made up of two H atoms each.
– Water, on the other hand, is a molecular compound that contains hydrogen and oxygen in a ratio of two H atoms and one O atom.
– Like atoms, molecules are electrically neutral.
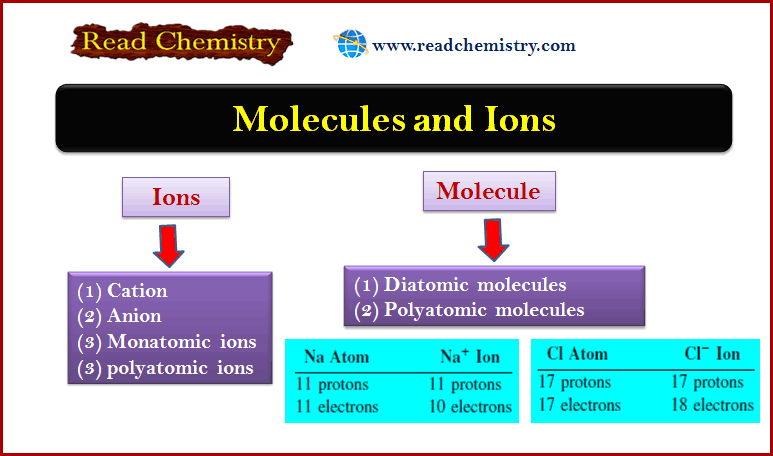
Diatomic molecules
– The hydrogen molecule, symbolized as H2 is called a diatomic molecule because it contains only two atoms.
– Other elements that normally exist as diatomic molecules are nitrogen (N2) and oxygen (O2), as well as the Group 7A elements—fluorine (F2), chlorine (Cl2), bromine (Br2), and iodine (I2).
– Of course, a diatomic molecule can contain atoms of different elements. Examples are hydrogen chloride (HCl) and carbon monoxide (CO).
Polyatomic molecules
– The vast majority of molecules contain more than two atoms.
– They can be atoms of the same element, as in ozone (O3), which is made up of three atoms of oxygen, or they can be combinations of two or more different elements.
– Molecules containing more than two atoms are called polyatomic molecules.
– Like ozone, water (H2O) and ammonia (NH3) are polyatomic molecules.
Ions
– An ion is an atom or a group of atoms that has a net positive or negative charge.
– The number of positively charged protons in the nucleus of an atom remains the same during ordinary chemical changes (called chemical reactions), but negatively charged electrons may be lost or gained.
Cation
– The loss of one or more electrons from a neutral atom results in a cation, an ion with a net positive charge.
– For example, a sodium atom (Na) can readily lose an electron to become a sodium cation, which is represented by Na+:
Anion
– On the other hand, an anion is an ion whose net charge is negative due to an increase in the number of electrons.
– A chlorine atom (Cl), for instance, can gain an electron to become the chloride ion Cl–:
– Sodium chloride (NaCl), ordinary table salt, is called an ionic compound because it is formed from cations and anions.
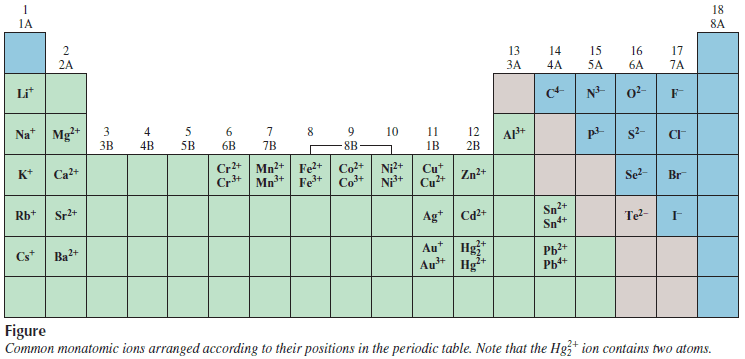
Monatomic ions
– An atom can lose or gain more than one electron.
– Examples of ions formed by the loss or gain of more than one electron are Mg2+, Fe3+, S2-, and N3-.
– These ions, as well as Na+ and Cl–, are called monatomic ions because they contain only one atom.
– The figure below shows the charges of several monatomic ions.
– With very few exceptions, metals tend to form cations and nonmetals form anions.
Polyatomic ions
– In addition, two or more atoms can combine to form an ion that has a net positive or net negative charge.
– Polyatomic ions such as OH– (hydroxide ion), CN– (cyanide ion), and NH4+ (ammonium ion) are ions containing more than one atom.
Reference: General Chemistry: The Essential Concepts / Raymond Chang, Jason Overby. ( sixth edition).