Nomenclature of Ionic Compounds
– In this subject, we will discuss the Nomenclature of Ionic Compounds.
Naming Compounds
– In addition to using formulas to show the composition of molecules and compounds, chemists have developed a system for naming substances based on their composition.
– we divided naming substances into three categories:
(1) Nomenclature of ionic compounds.
(2) Nomenclature of molecular compounds.
(3) Nomenclature of acids and bases.
– In this lesson, we will talk about the Nomenclature of Ionic Compounds
Nomenclature of Ionic Compounds
– In the lesson (Molecules and Ions) we learned that ionic compounds are made up of cations (positive ions) and anions (negative ions).
– With the important exception of the ammonium ion, NH4+, all cations of interest to us are derived from metal atoms.
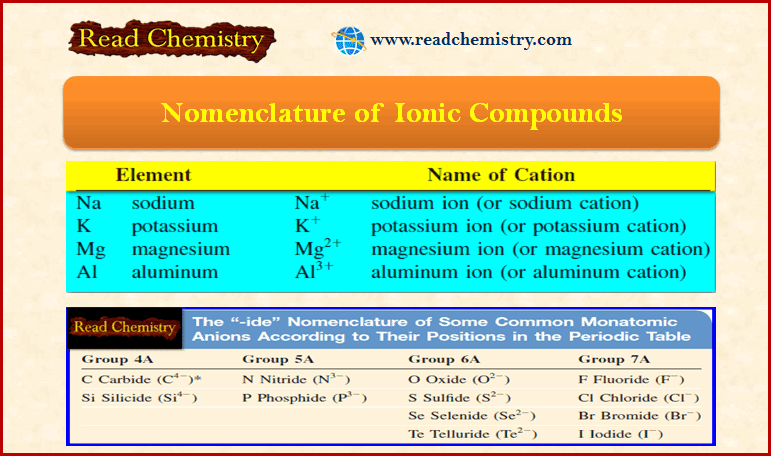
– Metal cations take their names from the elements. For example:
Binary ionic compounds
– Many ionic compounds are binary compounds or compounds formed from just two elements.
– For binary ionic compounds, the first element named is the metal cation, followed by the nonmetallic anion.
– Thus, NaCl is sodium chloride.
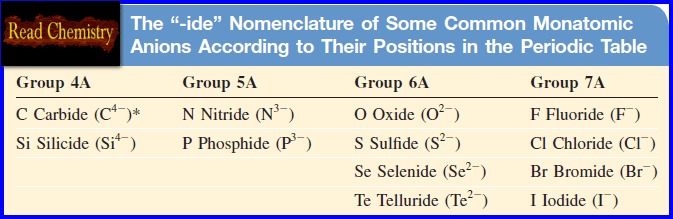
– The anion is named by taking the first part of the element name (chlorine) and adding “-ide.”
– Potassium bromide (KBr), zinc iodide (ZnI2), and aluminum oxide (Al2O3) are also binary compounds.
– The table below shows the “-ide” nomenclature of some common monatomic anions according to their positions in the periodic table:
Ternary ionic compounds
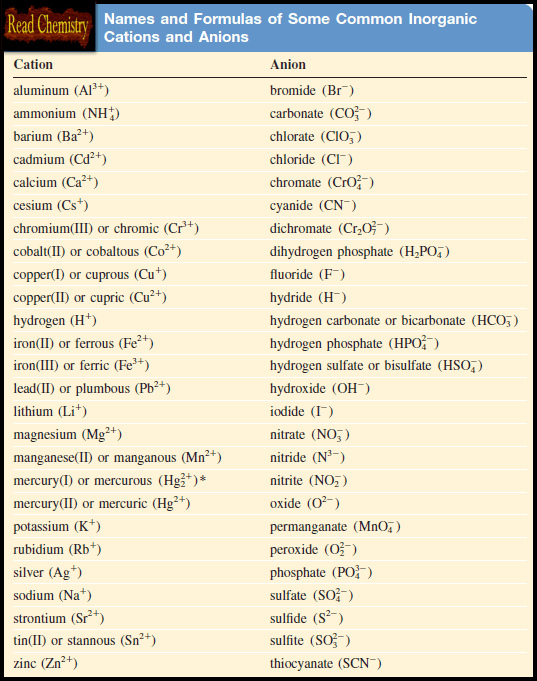
– The “-ide” ending is also used for certain anion groups containing different elements, such as hydroxide (OH–) and cyanide (CN–).
– Thus, the compounds LiOH and KCN are named lithium hydroxide and potassium cyanide.
– These and several other ionic substances are called ternary compounds, meaning compounds consisting of three elements.
– The table below lists alphabetically the names of several common cations and anions:
Ionic compounds of transition metals

– Certain metals, especially transition metals, can form more than one type of cation.
– Take iron as an example. Iron can form two cations: Fe2+ and Fe3+.
– The accepted procedure for designating different cations of the same element is to use Roman numerals.
– The Roman numeral I is used for one positive charge, II for two positive charges, and so on. This is called the Stock system.
– In the Stock system, the Fe2+ and Fe3+ ions are called iron(II) and iron(III), and the compounds FeCl2 (containing the Fe2+ ion) and FeCl3 (containing the Fe3+ ion) are called iron-two chloride and iron-three chloride, respectively.
– As another example, manganese (Mn) atoms can assume several different positive charges:

– These compound names are pronounced “manganese-two oxide,” “manganese-three oxide,” and “manganese four oxide.”
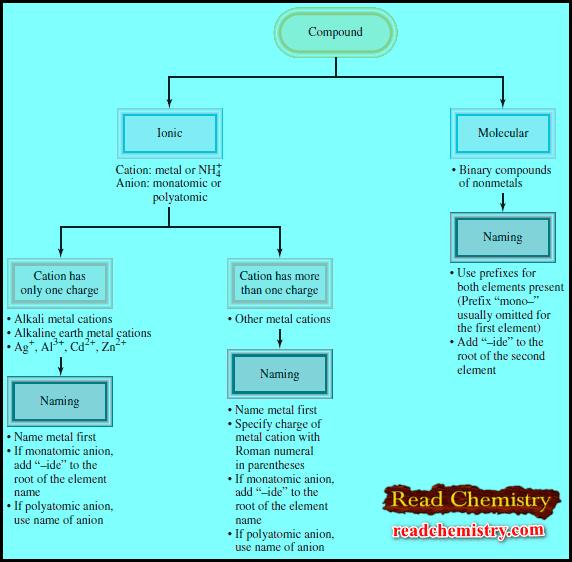
– The Figure blow summarizes the steps for naming ionic and molecular compounds:
Solved problems
Ex (1): Name the following compounds: (a) Fe(NO3)2, (b) Na2HPO4, and (c) (NH4)2SO3.
solution
(a) The nitrate ion (NO3–) bears one negative charge, so the iron ion must have two positive charges.
– Because iron forms both Fe2+ and Fe3+ ions, we need to use the Stock system and call the compound iron(II) nitrate.
(b) The cation is Na+ and the anion is HPO42- (hydrogen phosphate).
– Because sodium only forms one type of ion (Na+), there is no need to use sodium(I) in the name.
– The compound is sodium hydrogen phosphate.
(c) The cation is NH4+ (ammonium ion) and the anion is SO32- (sulfite ion).
– The compound is ammonium sulfite.
Ex (2): Write chemical formulas for the following compounds: (a) mercury(I) nitrate, (b) cesium oxide, and (c) strontium nitride.
solution
(a) The Roman numeral shows that the mercury ion bears a +1 charge.
– the mercury(I) ion is diatomic (that is, Hg2+2 ) and the nitrateion is NO3–.
– Therefore, the formula is Hg2(NO3)2.
(b) Each oxide ion bears two negative charges, and each cesium ion bears one positive charge (cesium is in Group 1A, as is sodium). Therefore, the formula is Cs2O.
(c) Each strontium ion (Sr2+) bears two positive charges, and each nitride ion (N3-) bears three negative charges.
– To make the sum of the charges equal zero, we must adjust the numbers of cations and anions:
3(+2) + 2(-3) = 0
– Thus, the formula is Sr3N2.
Reference: General Chemistry: The Essential Concepts / Raymond Chang, Jason Overby. ( sixth edition) .