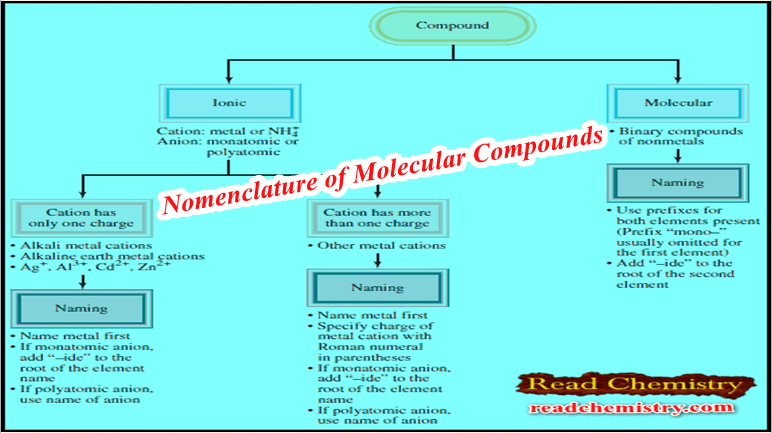
Dalton’s Atomic Theory: Definition, Statement, and Postulates
– In this subject, we will discuss the Dalton’s Atomic Theory: Definition, Statement, and Postulates
Atomic Theory
– In this lesson, we will explain atomic Theory especially, Dalton’s atomic theory.
– In the fifth-century b.c., the Greek philosopher Democritus expressed the belief that all matter consists of very small, indivisible particles. he named atomos (meaning uncuttable or indivisible).
– Although Democritus’ idea was not accepted by many of his contemporaries (notably Plato and Aristotle), somehow it endured.
– Experimental evidence from early scientific investigations provided support for the notion of “atomism” and gradually gave rise to the modern definitions of elements and compounds.
– It was in 1808 that an English scientist and schoolteacher, John Dalton, formulated a precise definition of the indivisible building blocks of matter that we call atoms.
Dalton’s atomic theory
– Dalton’s work marked the beginning of the modern era of chemistry.
– The hypotheses about the nature of matter on which Dalton’s atomic theory is based can be summarized as:
(1) Elements are composed of extremely small particles, called atoms.
(2) All atoms of a given element are identical, having the same size, mass, and chemical properties.
– The atoms of one element are different from the atoms of all other elements.
(3) Compounds are composed of atoms of more than one element.
– In any compound, the ratio of the number of atoms of any two of the elements present is either an integer or a simple fraction.
(4) A chemical reaction involves only the separation, combination, or rearrangement of atoms.
– it does not result in their creation or destruction.
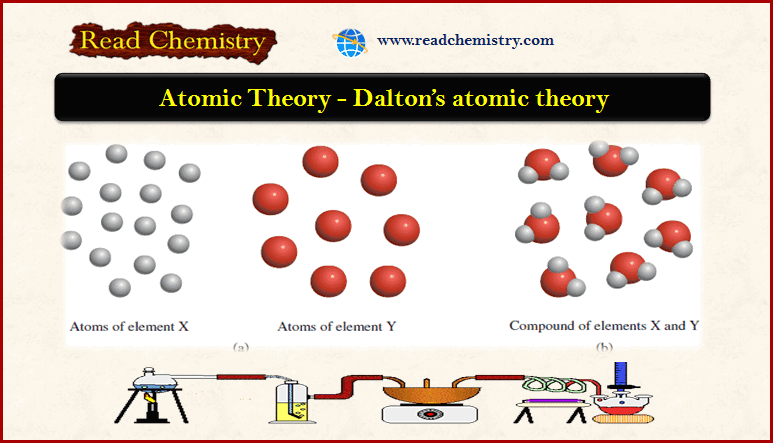
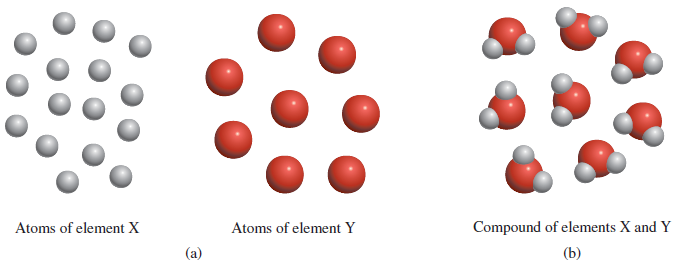
– In this figure:
(a) According to Dalton’s atomic theory, atoms of the same element are identical, but atoms of one element are different from atoms of other elements.
(b) Compounds formed from atoms of elements X and Y.
– In this case, the ratio of the atoms of element X to the atoms of element Y is 2:1.
– The Figure above is a schematic representation of hypotheses 2 and 3.
– Dalton’s concept of an atom was far more detailed and specific than Democritus’.
Dalton’s second hypothesis
– The second hypothesis states that atoms of one element are different from atoms of all other elements.
– Dalton did not attempt to describe the structure or composition of atoms. he had no idea what an atom was really like.
– However, he did realize that the different properties shown by elements such as hydrogen and oxygen can be explained by assuming that hydrogen atoms are not the same as oxygen atoms.
Dalton’s third hypothesis
– The third hypothesis suggests that to form a certain compound, we need not only atoms of the right kinds of elements. But the specific numbers of these atoms as well.
– This idea is an extension of a law published in 1799 by Joseph Proust, a French chemist.
– Proust’s law of definite proportions states that different samples of the same compound always contain its constituent elements in the same proportion by mass.
– Thus, if we were to analyze samples of carbon dioxide gas obtained from different sources, we would find in each sample the same ratio by mass of carbon to oxygen.
– It stands to reason, then, that if the ratio of the masses of different elements in a given compound is fixed, the ratio of the atoms of these elements in the compound also must be constant.
– Dalton’s third hypothesis also supports another important law, the law of multiple proportions.
– According to this law, if two elements can combine to form more than one compound, the masses of one element that combine with a fixed mass of the other element are in ratios of small whole numbers.
– Dalton’s theory explains the law of multiple proportions quite simply: The compounds differ in the number of atoms of each kind that combine.
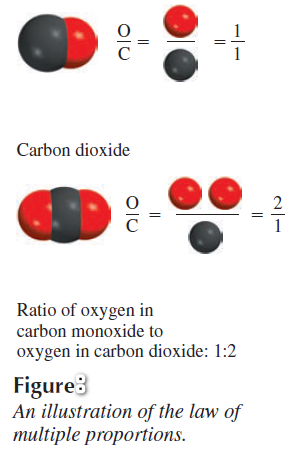
Example of the law of multiple proportions
– For example, carbon forms two stable compounds with oxygen, namely, carbon monoxide and carbon dioxide.
– Modern measurement techniques indicate that one atom of carbon combines with one atom of oxygen in carbon monoxide and that one atom of carbon combines with two oxygen atoms in carbon dioxide.
– Thus, the ratio of oxygen in carbon monoxide to oxygen in carbon dioxide is 1:2.
– This result is consistent with the law of multiple proportions because the mass of an element in a compound is proportional to the number of atoms of the element present.
Dalton’s fourth hypothesis
– Dalton’s fourth hypothesis is another way of stating the law of conservation of mass, which is that matter can be neither created nor destroyed.
– Because matter is made of atoms that are unchanged in a chemical reaction, it follows that mass must be conserved as well.
– Dalton’s brilliant insight into the nature of matter was the main stimulus for the rapid progress of chemistry during the nineteenth century.
Conclusion of Dalton’s atomic theory
(1) we can define an atom as the basic unit of an element that can enter into a chemical combination.
(2) Dalton imagined an atom that was both extremely small and indivisible.
– However, a series of investigations that began in the 1850s and extended into the twentieth century demonstrated that atoms possess internal structure.
(3) Atoms are made up of even smaller particles, which are called subatomic particles.
– This research led to the discovery of three such particles—electrons, protons, and neutrons.
Reference: General Chemistry: The Essential Concepts / Raymond Chang, Jason Overby. ( sixth edition).