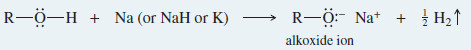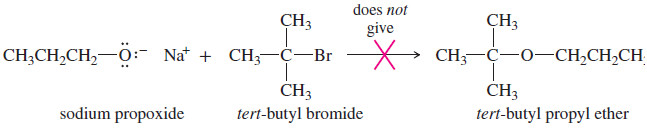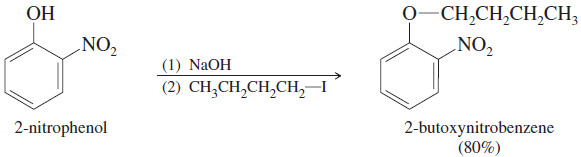Williamson Ether Synthesis : Mechanism, Examples
– In this topic, we will discuss the Williamson Ether Synthesis : Mechanism, Examples and Solved problems.
Williamson Ether Synthesis
– We have already seen most of the common methods for synthesizing ethers.
– We review them at this time, looking more closely at the mechanisms to see which methods are most suitable for preparing various kinds of ethers.
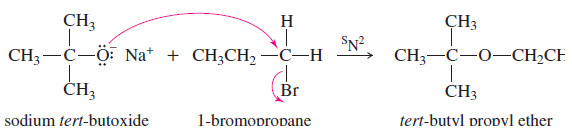
– The Williamson ether synthesis is the most reliable and versatile ether synthesis.
– This method involves the attack of an alkoxide ion on an unhindered primary alkyl halide or tosylate.
– Some alcohols react sluggishly with both sodium and potassium.
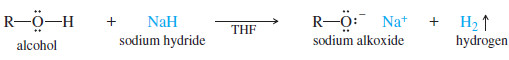
– In these cases, a useful alternative is sodium hydride, usually in tetrahydrofuran solution.
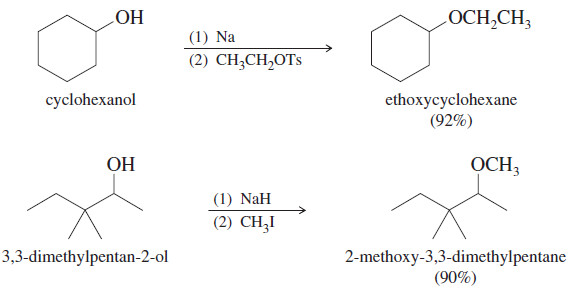
– Sodium hydride reacts quickly to form the alkoxide, even with difficult compounds.
– The alkoxide ion is a strong nucleophile as well as a powerful base.
– Unlike the alcohol itself, the alkoxide ion reacts with primary alkyl halides and tosylates to form ethers.
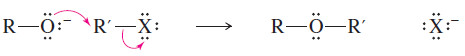
– This general reaction, called the Williamson ether synthesis, is an SN2 displacement.
– The alkyl halide (or tosylate) must be primary so that a back-side attack is not hindered.
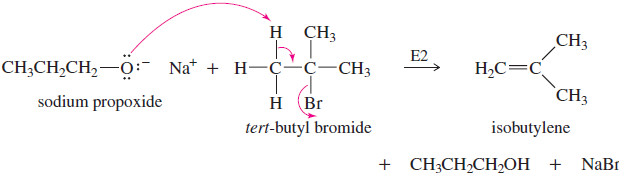
– When the alkyl halide is not primary, elimination usually results
– Secondary alkyl halides and tosylates are occasionally used in the Williamson synthesis, but elimination competes, and the yields are often poor.
– The alkoxide is commonly made by adding Na, K, or NaH to the alcohol.
Williamson Ether Synthesis Mechanism
– This is the most important method for making ethers.
Step (1): Form the alkoxide of the alcohol having the more hindered group.
Step (2): The alkoxide displaces the leaving group of a good SN2 substrate.
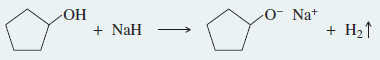
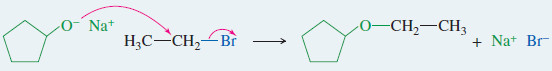
Example: Synthesis of cyclopentyl ethyl ether
Step (1): Form the alkoxide of the alcohol with the more hindered group.
Step (2): The alkoxide displaces the leaving group of a good SN2 substrate.
– In the Williamson ether synthesis, the alkyl halide (or tosylate) must be a good SN2 substrate (usually primary).
– In proposing a Williamson synthesis, we usually choose the less hindered alkyl group to be the halide (or tosylate) and the more hindered group to be the alkoxide ion.
Examples of Williamson Ether Synthesis
Solved Problem
(a) Why is the following reaction a poor method for the synthesis of tert-butyl propyl ether?
(b) What would be the major product from this reaction?
(c) Propose a better synthesis of tert-butyl propyl ether.
Solution
(a) The desired SN2 reaction cannot occur on the tertiary alkyl halide.
(b) The alkoxide ion is a strong base as well as a nucleophile, and elimination prevails.
(c) A better synthesis would use the less hindered alkyl group as the SN2 substrate and the alkoxide of the more hindered alkyl group.
Synthesis of Phenyl Ethers
– A phenol (aromatic alcohol) can be used as the alkoxide fragment, but not the halide fragment, for the Williamson ether synthesis.
– Phenols are more acidic than aliphatic alcohols, and sodium hydroxide is sufficiently basic to form the phenoxide ion.
– As with other alkoxides, the electrophile should have an unhindered primary alkyl group and a good leaving group.
References:
- Organic chemistry / L.G. Wade, Jr / 8th ed, 2013 / Pearson Education, Inc. USA.
- Fundamental of Organic Chemistry / John McMurry, Cornell University/ 8th ed, 2016 / Cengage Learningm, Inc. USA.
- Organic Chemistry / T.W. Graham Solomons, Craig B. Fryhle , Scott A. Snyder / 11 ed, 2014/ John Wiley & Sons, Inc. USA.