Alkene Synthesis by High-Temperature Industrial Methods
Alkene Synthesis by High-Temperature Industrial Methods
(1) Catalytic Cracking of Alkanes
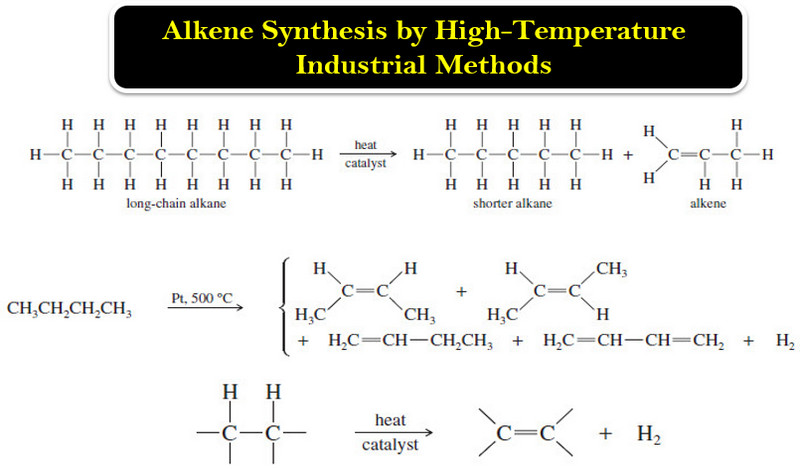
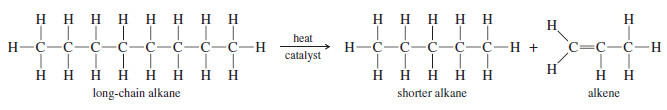
– The least expensive way to make alkenes on a large scale is by the catalytic cracking of petroleum: heating a mixture of alkanes in the presence of a catalyst (usually aluminosilicates).
– Alkenes are formed by bond cleavage to give an alkene and a shortened alkane
– Cracking is used primarily to make small alkenes, up to about six carbon atoms. Its value depends on having a market for all the different alkenes and alkanes produced.
– The average molecular weight and the relative amounts of alkanes and alkenes can be controlled by varying the temperature, catalyst, and concentration of hydrogen in the cracking process.
– A careful distillation on a huge column separates the mixture into its pure components, ready to be packaged and sold.
– Because the products are always mixtures, catalytic cracking is unsuitable for laboratory synthesis of alkenes.
– Better methods are available for synthesizing relatively pure alkenes from a variety of other functional groups.
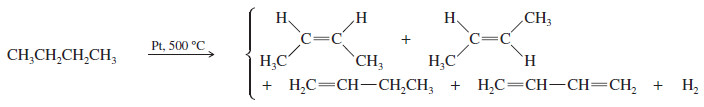
(2) Alkene Synthesis by Dehydrogenation of Alkanes
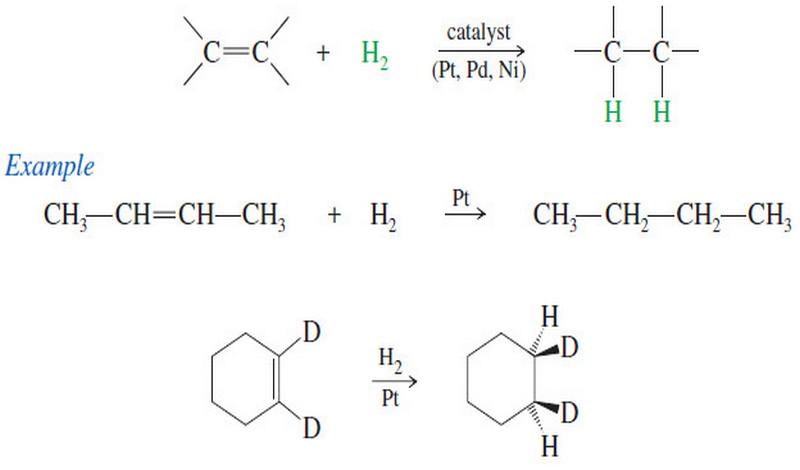
– Dehydrogenation is the removal of H2 from a molecule, just the reverse of hydrogenation.
– Dehydrogenation of an alkane gives an alkene.
– This reaction has an unfavorable enthalpy change but a favorable entropy change.
ΔH° = +80 to +120 kJ/mol (+20 to +30 kcal/mol)
ΔS° = +125 J/kelvin-mol
– The hydrogenation of alkenes is exothermic, with values of ΔH° around -80 to -120kj/mol (-20 to -30kj/mol ).
– Therefore, dehydrogenation is endothermic and has an unfavorable (positive) value of ΔH°
– The entropy change for dehydrogenation is strongly favorable ΔS° = +120 J/kelvin-mol), however, because one alkane molecule is converted into two molecules (the alkene and hydrogen), and two molecules are more disordered than one.
– The equilibrium constant for the hydrogenation–dehydrogenation equilibrium depends on the change in free energy, ΔG = ΔH – TΔS.
– At room temperature, the enthalpy term predominates and hydrogenation is favored.
– When the temperature is raised, however, the entropy term (- TΔS) becomes larger and eventually dominates the expression.
– At a sufficiently high temperature, dehydrogenation is favored
– In many ways, dehydrogenation is similar to catalytic cracking.
– In both cases, a catalyst lowers the activation energy, and both reactions use high temperatures to increase a favorable entropy term (- TΔS) and overcome an unfavorable enthalpy term (ΔH).
– Unfortunately, dehydrogenation and catalytic cracking also share a tendency to produce mixtures of products, and neither reaction is well suited for the laboratory synthesis of alkenes.