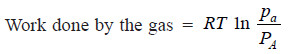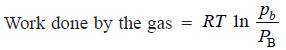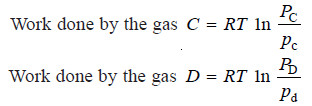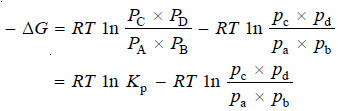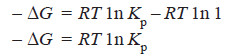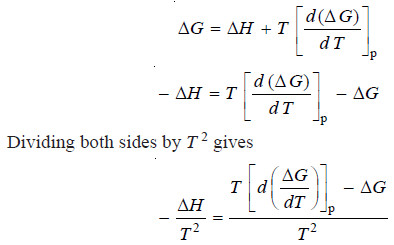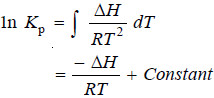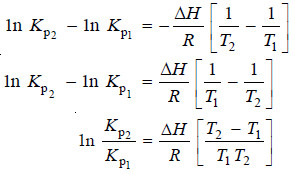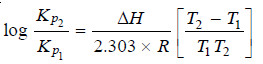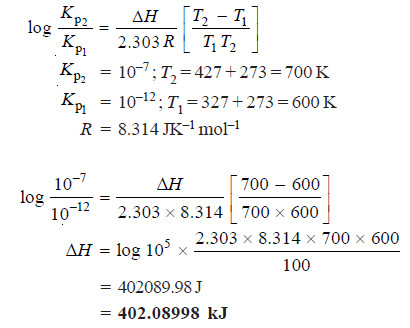Van’t Hoff isotherm – Van’t Hoff Isochore
Van’t Hoff isotherm
– The van’t Hoff isotherm gives the net work that can be obtained from a gaseous reactant at constant temperature when both the reactants and the products are at suitable arbitrary pressures.
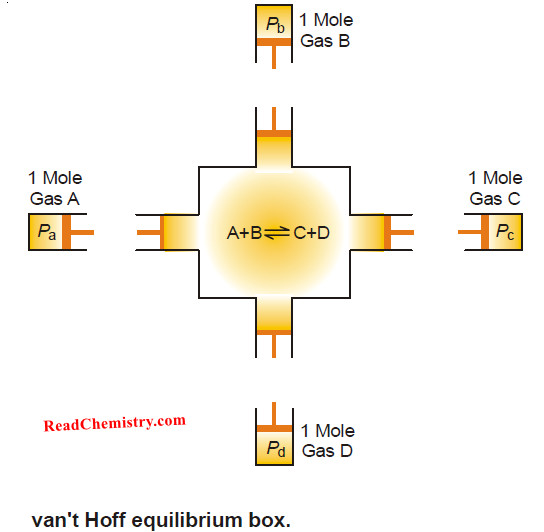
– It can be derived by using the “equilibrium box” which is a theoretical device with the supposition that of its four walls, one is permeable to A, the second to B, the third to C and the fourth to D when the gaseous reaction to be considered is
A + B ↔ C + D
– Let the initial pressures of A and B be pa and pb and the final pressure of C and D be pc and pd respectively and let the equilibrium pressure of the four be PA, PB, PC and PD respectively.
– The following theoretical operations may be performed:
(1) Change the pressure on A from the initial pressure pa to the equilibrium pressure PA.
(2) Change the pressure on B from pb to PB.
(3) Introduce 1 gm mole of A and 1 gm mole of the gas B through their respective semipermeable membranes into the equilibrium box which contains the reactants and the products at the equilibrium pressure.
– This will not involve any work as the partial pressures of A and B inside the box are equal to the pressure of the gases coming in.
– A and B react to form 1 gm mole of C and 1 gm mole of D.
(4) Withdraw 1 gm mole of C and 1 gm mole of D from the equilibrium box through their respective semipermeable walls.
– No work is done in this process as the gases come out at the equilibrium pressure PC and PD.
(5) Now alter the pressure on the gas from the equilibrium pressure PC and PD to the final pressure pc and pd.
– As the change in free energy is equal to the total work done by the gases:
– If the reaction is started with reactants at a partial pressure of 1 atmosphere and the resulting products are also at 1 atmosphere pressure we have,
i.e., the net work of the reaction is equal to the decrease in free energy of the system and is given by the expression, RT ln Kp or 2.303 RT log Kp.
– It will be observed that ΔG is + ve when Kp is less than unity.
– It is – ve when Kp is greater than one and is zero when Kp = 1.
Van’t Hoff Isochore
– The van’t Hoff Isochore is obtained by combining the van’t Hoff Isotherm with the Gibbs Helmholtz equation.
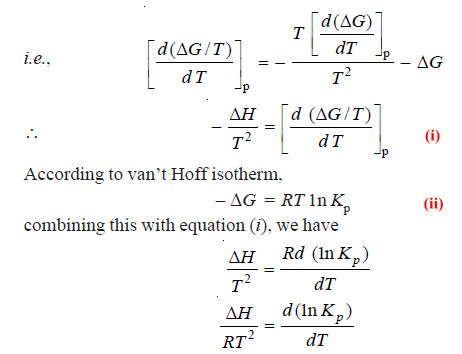
– The right-hand side of the above expression is obtained by differentiating ΔG/T w.r.t. T at constant pressure:
– The above equation is known as van’t Hoff Isochore.
– For applying the Isochore to any particular reaction, it is essential to integrate it.
– If ΔH remains constant over a range of temperature, we have on integration:
– Applying the limits T1 and T2 at which the equilibrium constants are Kp1 and Kp2 respectively, we have:
or
– Knowing the equilibrium constant at two different temperatures it is possible, therefore, to calculate the change in heat content.
Solved problem
The equilibrium constant Kp for a reaction A + B ↔ C + D is 10–12 at 327ºC and 10–7 at 427ºC. Calculate the enthalpy of the reaction. (R = 8.314 JK– mol–)
Solution
Applying van’t Hoff’s equation
References
- Atkins’ Physical Chemistry / Peter Atkin, Julio de Paula, James Keeler / 12th edition, 2022 / Oxford University Press, UK.
- Physical Chemistry/ Robert G. Mortimer/ 3rd Edition / 2008/ Elsevier Inc, USA.
- Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolor edition/ S. Chand Publishing co / india.
- Physical chemistry for the chemical sciences / Raymond Chang, John W. Thoman, Jr./1st edition, 2014/ University Science Books, USA