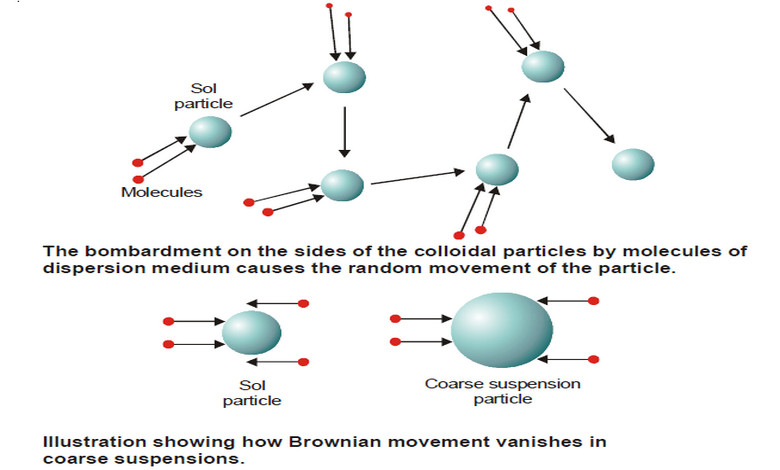
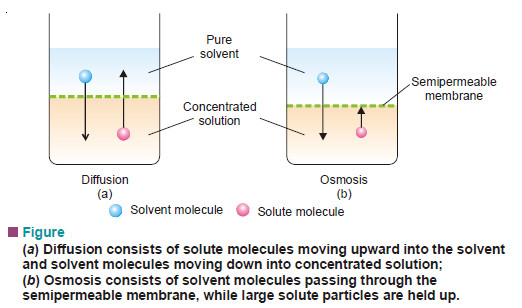
Concise Physical Chemistry book by Donald W.Rogers
– In this subject, we will discuss the free download of Concise Physical Chemistry book by Donald W.Rogers

The Preface of Concise Physical Chemistry book
– Physical chemistry stands at the intersection of the power and generality of classical and quantum physics with the minute molecular complexity of chemistry and biology.
– Any molecular process that can be envisioned as a flow from a higher energy state to a lower state is subject to analysis by the methods of classical thermodynamics.
– Chemical thermodynamics tells us where a process is going. Chemical kinetics tells us how long it will take to get there.
– Evidence for and application of many of the most subtle and abstract principles of quantum mechanics are to be found in the physical interpretation of chemical phenomena.
– The vast expansion of spectroscopy from line spectra of atoms well known in the nineteenth century to the magnetic resonance imaging (MRI) of today’s diagnostic procedures is a result of our gradually enhanced understanding of the quantum mechanical interactions of energy with simple atomic or complex molecular systems.
– Mathematical methods developed in the domain of physical chemistry can be successfully applied to very different phenomena.
– In the study of seemingly unrelated phenomena, we are astonished to find that electrical potential across a capacitor, the rate of isomerization of cyclopentene, and the growth of marine larvae either as individuals or as populations have been successfully modeled by the same first-order differential equation.
– Many people in diverse fields use physical chemistry but do not have the opportunity to take a rigorous three-semester course or to master one of the several∼1000-page texts in this large and diverse field.
– Concise Physical Chemistry is intended to meet (a) the needs of professionals in fields other than physical chemistry who need to be able to master or review a limited portion of physical chemistry or (b) the need of instructors who require a manageable text for teaching a one-semester course in the essentials of the subject.
– The present text is not, however, a diluted form of physical chemistry.
– Topics are treated as brief, self-contained units, graded in difficulty from reintroduction to some of the concepts of general chemistry in the first few chapters to research-level computer applications in the later chapters.
Description of Concise Physical Chemistry book
Book name: Concise Physical Chemistry book by Donald W. Rogers.
Author: Donald W. Rogers
Department of Chemistry and Biochemistry, The Brooklyn Center, Long Island University, Brooklyn, NY
Edition: Unkown
Year: 2011
No of Chapters: 21
No of Pages: 405
File format: pdf
File size: 2.4 MB
Contents of the book
Chapter 1: Ideal Gas Laws
Chapter 2: Real Gases: Empirical Equations
Chapter 3: The Thermodynamics of Simple Systems
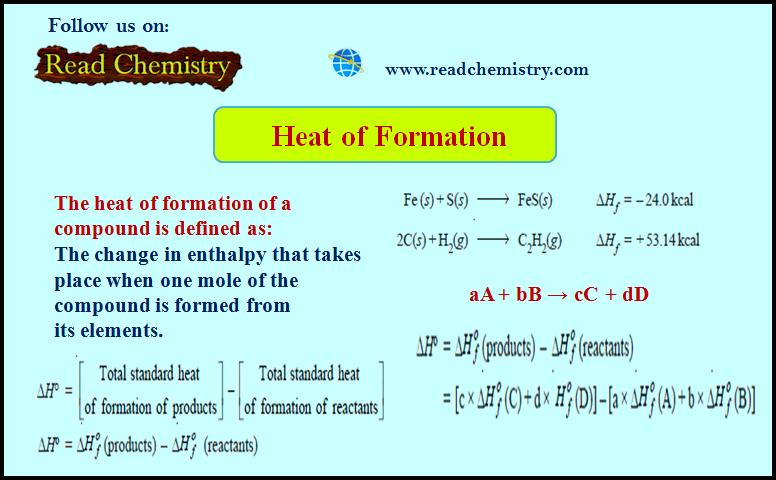
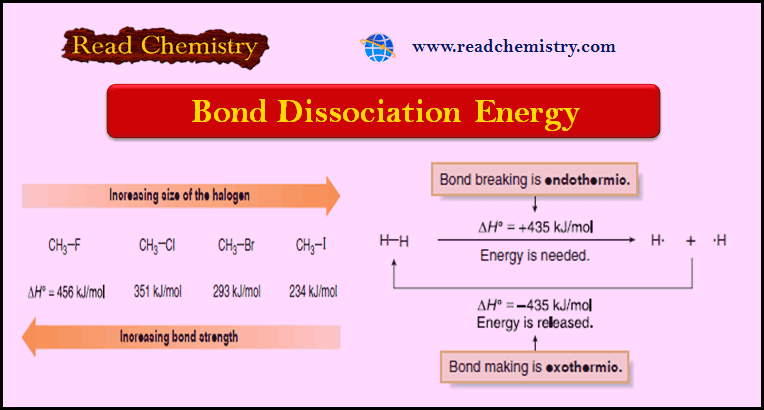
Chapter 4: Thermochemistry
Chapter 5: Entropy and the Second Law
Chapter 6: The Gibbs Free Energy
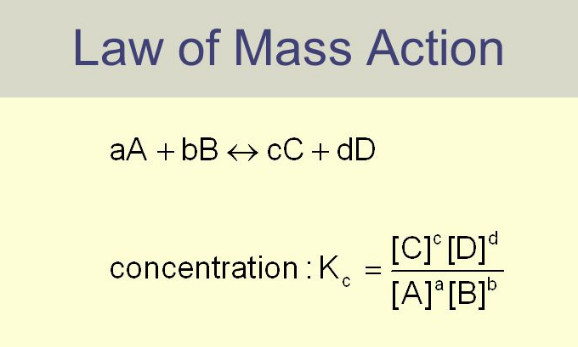
Chapter 7: Equilibrium
Chapter 8: A Statistical Approach to Thermodynamics
Chapter 9: The Phase Rule
Chapter 10: Chemical Kinetics
Chapter 11: Liquids and Solids
Chapter 12: Solution Chemistry
Chapter 13: Coulometry and Conductivity
Chapter 14: Electrochemical Cells
Chapter 15: Early Quantum Theory: A Summary
Chapter 16: Wave Mechanics of Simple Systems
Chapter 17: The Variational Method: Atoms
Chapter 18: Experimental Determination of Molecular Structure
Chapter 19: Classical Molecular Modeling
Chapter 20: Quantum Molecular Modeling
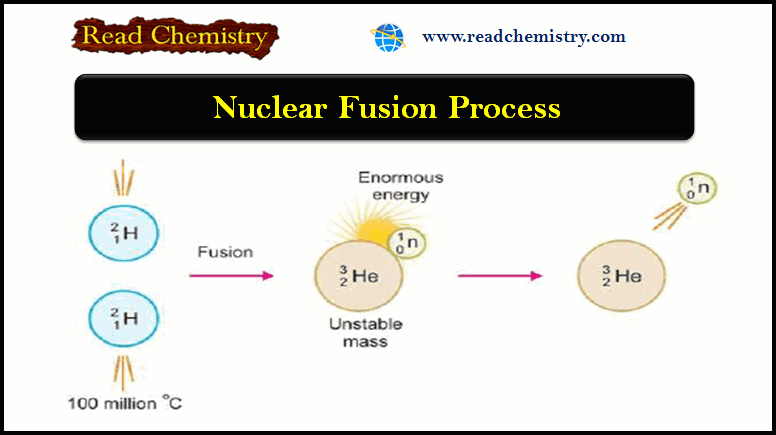
Chapter 21: Photochemistry and the Theory of Chemical Reactions
Cover of the book
Free Download of Concise Physical Chemistry book
File format: pdf
File size: 2.4 MB
For more free books. Visit Category: Free books