Thermodynamic Processes
– Thermodynamic Processes involve the change of conditions (temperature, pressure, and volume).
Thermodynamic Processes
– When a thermodynamic system changes from one state to another, the operation is called a Process.
– Thermodynamic Processes involve the change of conditions (temperature, pressure, and volume).
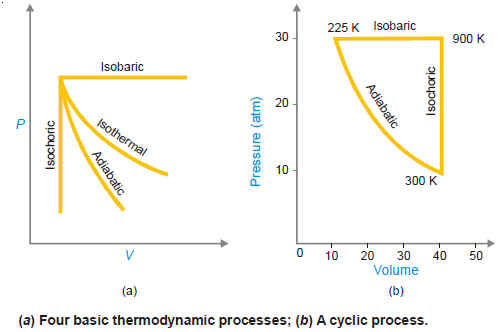
– The various types of thermodynamic processes are:
(1) Isothermal Processes
(2) Adiabatic Processes
(3) Isobaric Processes
(4) Isochoric Processes
(5) Cyclic Process
(1) Isothermal Processes
– Those processes in which the temperature remains fixed, are termed isothermal processes.
– This is often achieved by placing the system in a thermostat (a constant temperature bath).
– For an isothermal process dT = 0
(2) Adiabatic Processes
– Those processes in which no heat can flow into or out of the system, are called adiabatic processes.
– Adiabatic conditions can be approached by carrying the process in an insulated container such as ‘thermos’ bottle.
– High vacuum and highly polished surfaces help to achieve thermal insulation.
– For an adiabatic process dq = 0
(3) Isobaric Processes
– Those processes which take place at constant pressure are called isobaric processes.
– For example, the heating of water to its boiling point and its vaporization take place at the same atmospheric pressure.
– These changes are, therefore, designated as isobaric processes and are said to take place isobarically.
– For an isobaric process dp = 0
(4) Isochoric Processes
– Those processes in which the volume remains constant are known as isochoric processes.
– The heating of a substance in a non-expanding chamber is an example of an isochoric process.
– For isochoric processes dV = 0.
(5) Cyclic Process
– When a system in a given state goes through a number of different processes and finally returns to its initial state, the overall process is called a cycle or cyclic process.
– For a cyclic process dE = 0, dH = 0.
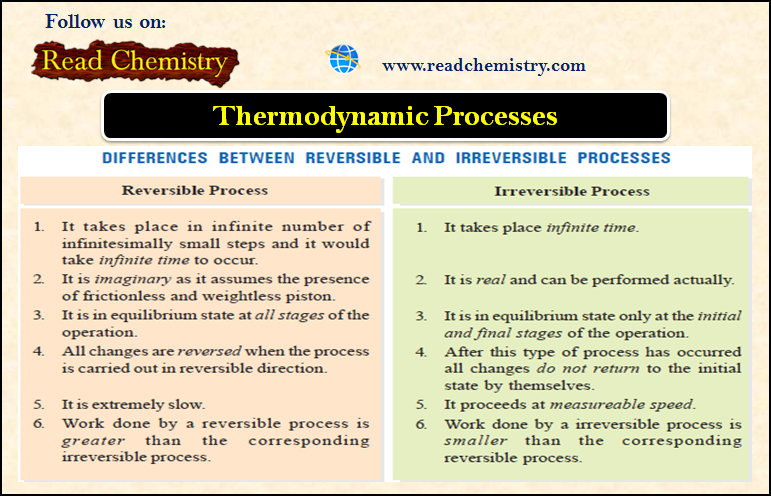
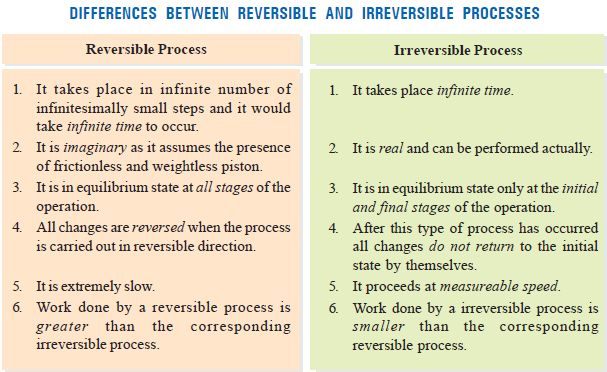
Reversible and Irreversible Processes
– A thermodynamic reverse process is one that takes place infinitesimally slowly and its direction at any point can be reversed by an infinitesimal change in the state of the system.
– In fact, a reversible process is considered to proceed from the initial state to the final state through an infinite series of infinitesimally small stages.
– At the initial, final, and all intermediate stages, the system is in an equilibrium state.
– This is so because an infinitesimal change in the state of the system at each intermediate step is negligible.
– When a process goes from the initial to the final state in a single step and cannot be carried in the reverse order, it is said to be an irreversible process.
Explanation with Example
– Here the system is in an equilibrium state in the beginning and at the end, but not at points in between.
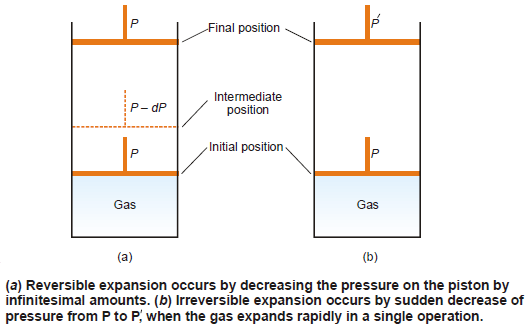
– Consider a certain quantity of gas contained in a cylinder having a weightless and frictionless piston.
– The expansion of the gas can be carried by two methods illustrated in the following figure:
– Let the pressure applied to the piston be P and this is equal to the internal pressure of the gas.
– Since the external and internal pressures are exactly counterbalanced, the piston remains stationary and there is no change in the volume of the gas.
– Now suppose the pressure on the piston is decreased by an infinitesimal amount of dP.
– Thus the external pressure on the piston being P – dP, the piston moves up and the gas will expand by an infinitesimal small amount.
– The gas will, therefore, be expanded infinitely slowly i.e., by a thermodynamically reversible process.
– At all stages in the expansion of the gas, dP being negligibly small the gas is maintained in a state of equilibrium throughout.
– If at any point of the process, the pressure is increased by dP, the gas would contract reversibly.
– On the other hand, the expansion is irreversible in the figure above (b) if the pressure on the piston is decreased suddenly.
– It moves upward rapidly in a single operation.
– The gas is in an equilibrium state in the initial and final stages only.
– The expansion of the gas, in this case, takes place in an irreversible manner.
Difference between Reversible and Irreversible Processes
Reference: Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolor edition.