General Chemistry
General Chemistry introduces basic chemical concepts, including atomic structure, bonding, reactions, stoichiometry, states of matter, and periodic trends. It lays the groundwork for advanced study in all chemistry branches.
-
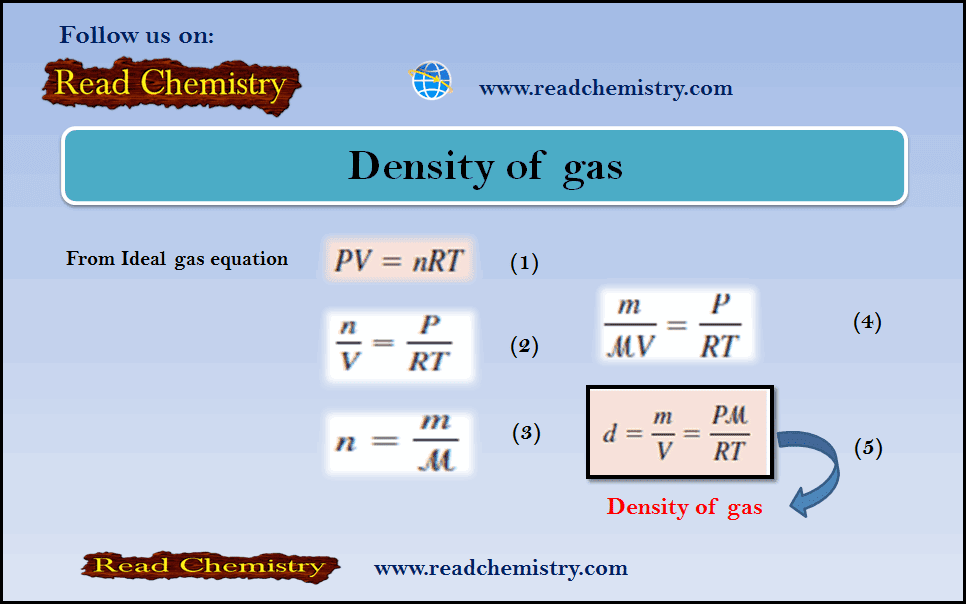
Density of gas: Definition, Equation, Solved Examples
– In this subject, we will discuss the Density of gas (Definition, Equation, Solved Examples) The Density of gas –…
Read More » -
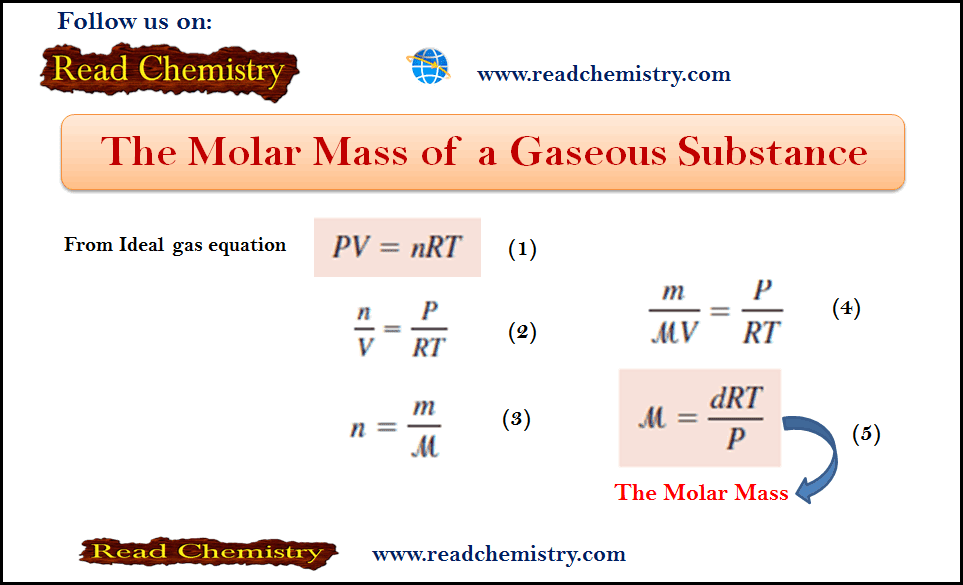
Molar Mass of gas: Definition, Equation, Solved Examples
– In this subject, we will discuss the Molar Mass of gas (Definition, Equation, Solved Examples) The Molar Mass of…
Read More » -
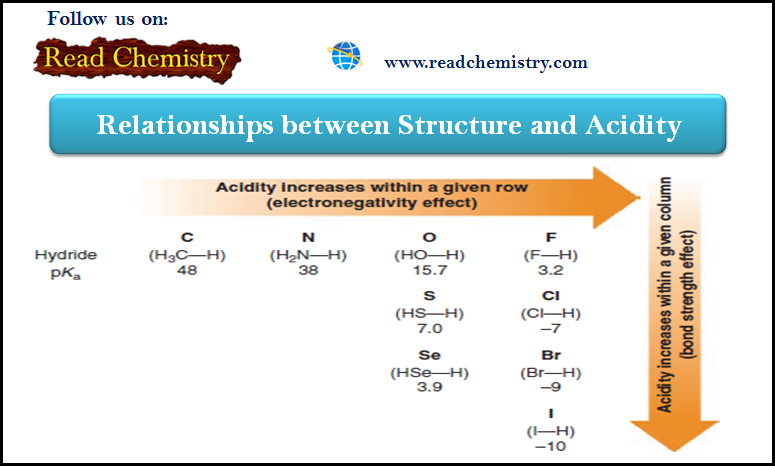
Acidity: The relationship between Structure and Acidity
– In this subject, we will discuss the Relationships between Structure and Acidity. – The strength of a Brønsted–Lowry acid…
Read More » -
How to Predict the Outcome of acid-base reaction
– In this subject, we will discuss How to Predict the Outcome of acid-base reaction. How To Predict the Outcome…
Read More » -
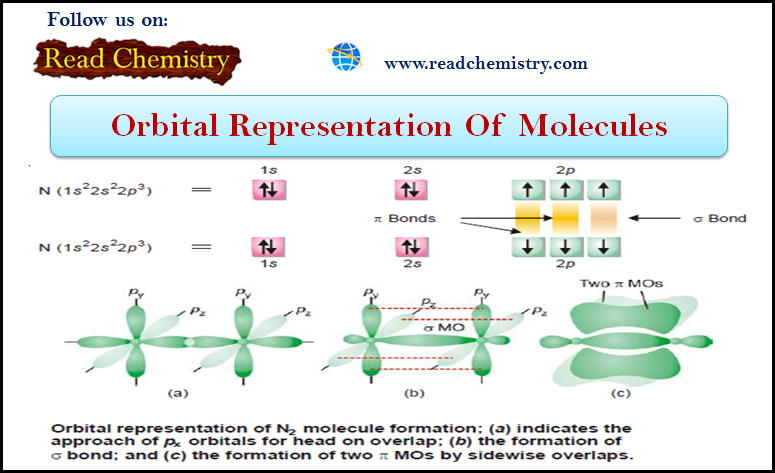
Orbital Representation of Molecules
– In this subject, we will discuss the Orbital Representation of some Molecules (1) Orbital Representation of H2 molecule –…
Read More » -
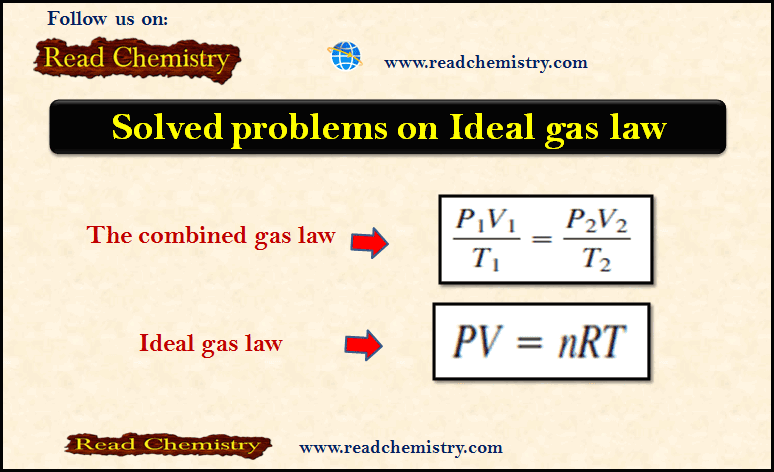
The Ideal gas law: Solved Problems
– In this subject, we will discuss Solved Problems on The Ideal gas law Problem (1) on The ideal gas…
Read More » -
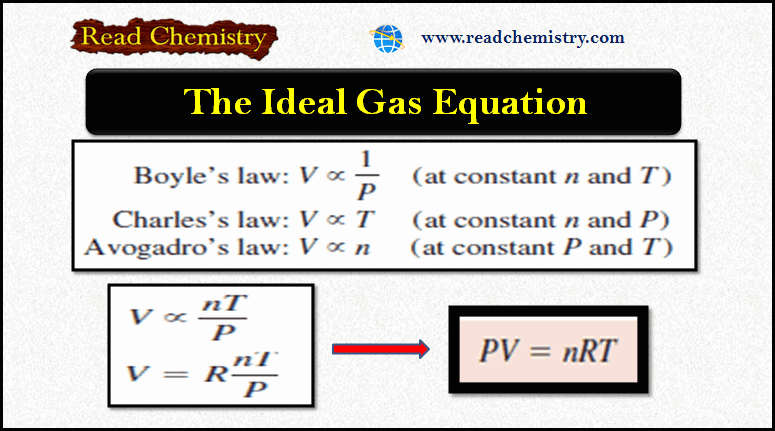
Ideal Gas Equation: Definition, Formula, Notes
– In this subject, we will discuss the Ideal Gas Equation (Definition, Formula, Notes) The Ideal Gas Equation – Let…
Read More » -
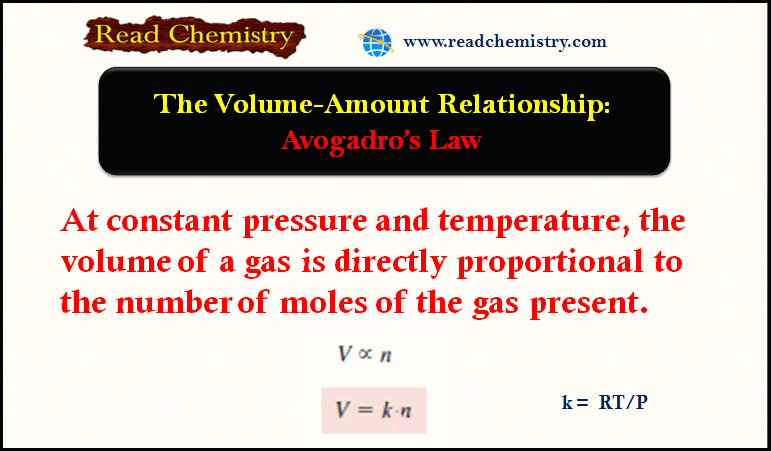
Avogadro’s Law: The Volume-Amount Relationship
– In this subject, we will discuss the Avogadro’s Law: The Volume-Amount Relationship Avogadro’s Law: The Volume-Amount Relationship – The…
Read More » -
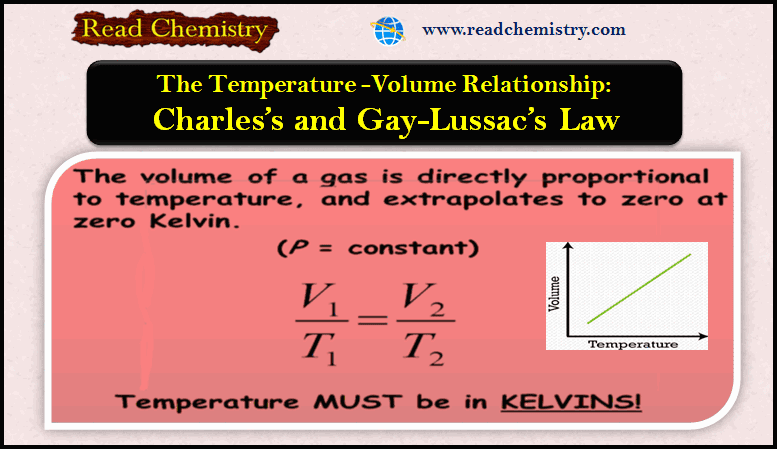
Charles’s Law: Relationship Between Temperature And Volume
– In this subject, we will discuss Charles’s Law: Relationship Between Temperature And Volume ( V-T relationship). Relationship Between Temperature…
Read More » -
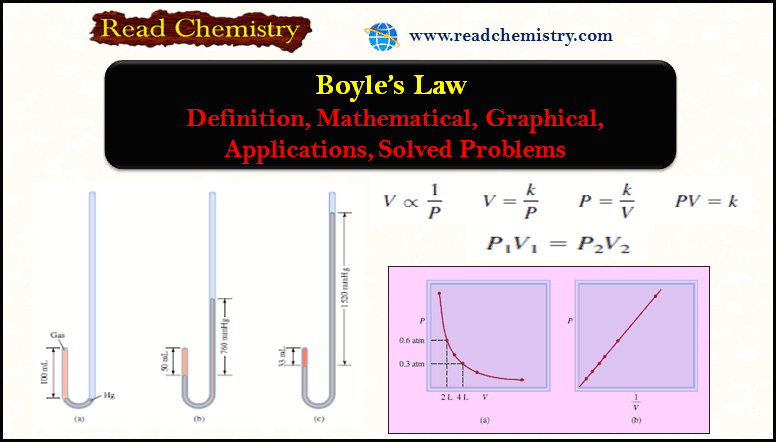
Boyle’s Law: Definition, Mathematical, Graphical, Applications
– In this subject, we will discuss Boyle’s Law: Definition, Mathematical, Graphical, Applications How did Boyle discover his Law? –…
Read More » -
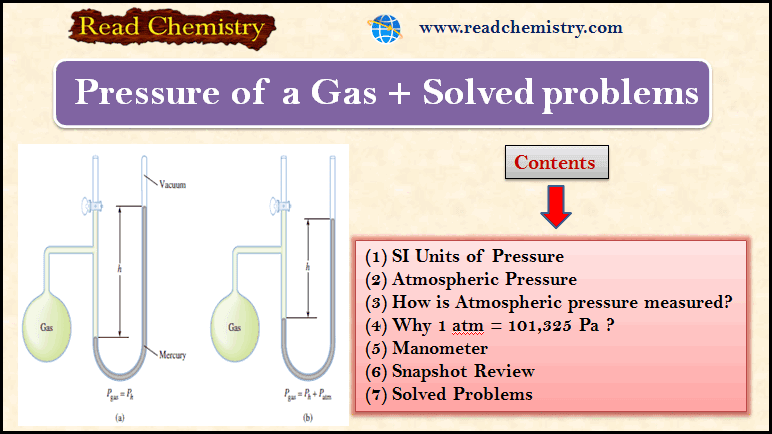
Gas Pressure: Definition, Formula and Solved problems
– In this subject, we will discuss Gas Pressure: Definition, Formula, and Solved problems Gas Pressure – Gases exert pressure…
Read More » -
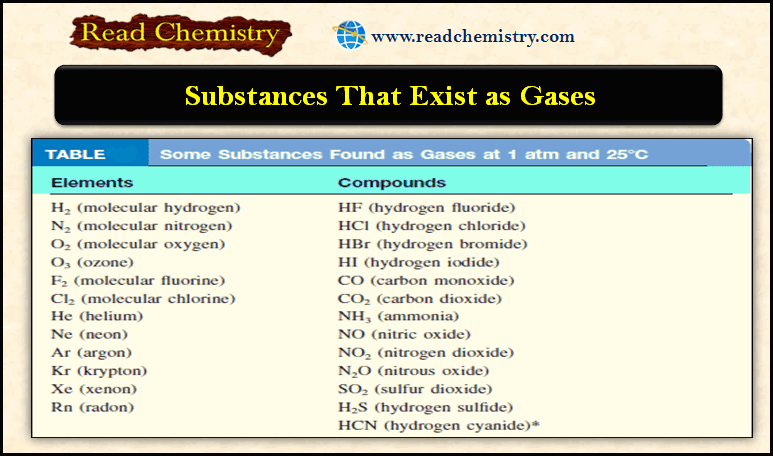
Gaseous Substances: Substances That Exist as Gases
– In this subject, we will discuss the Gaseous Substances: Substances That Exist as Gases Gaseous Substances – Under certain…
Read More » -
Redox Reactions: Types, Examples, Applications, Balancing
– In this subject, we will discuss the Redox Reactions: Types, Examples, Applications, Balancing. Types of Redox Reactions – Among…
Read More » -
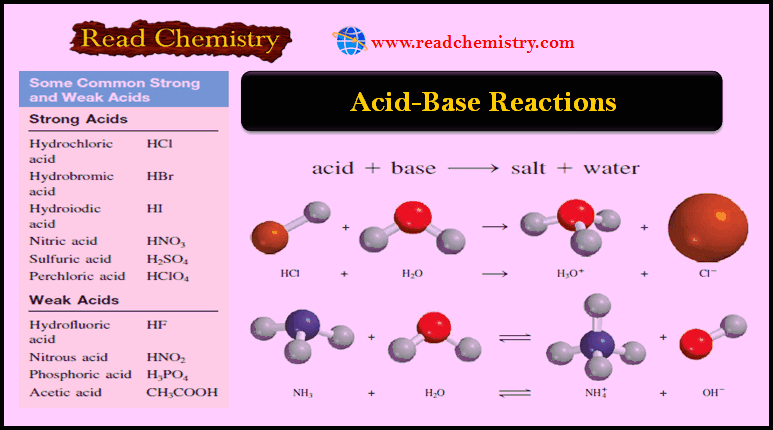
Acid-Base Reactions: Definition, Examples, and Uses
– In this subject, we will discuss the Acid-Base Reactions: Definition, Examples, and Uses – Acids and bases are as…
Read More » -
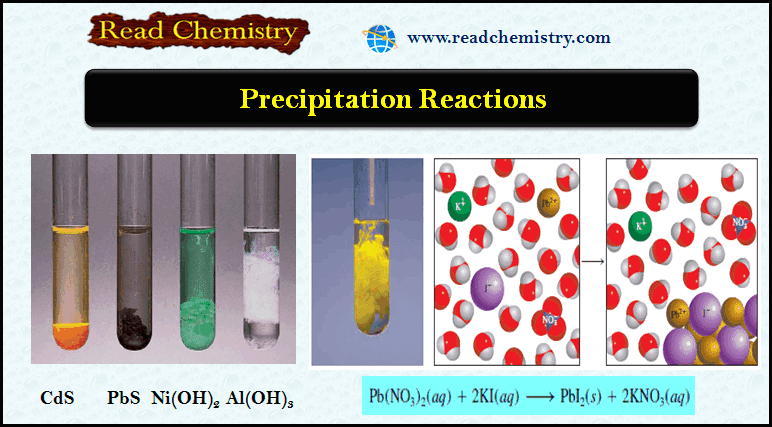
Precipitation Reactions: Definition and Examples
– In this subject, we will discuss the Precipitation Reactions: Definition and Examples What are Precipitation Reactions? – One common…
Read More » -
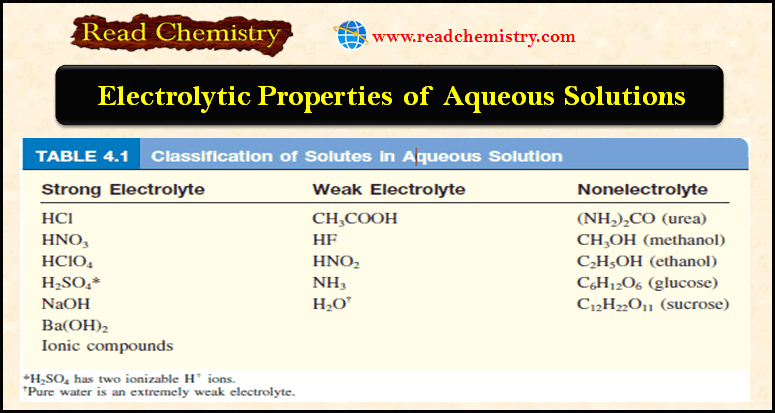
Aqueous Solutions: Definition, Examples, Electrolytic Properties
– In this subject, we will discuss the Aqueous Solutions: Definition, Examples, Electrolytic Properties Difference between Solution, solute, solvent –…
Read More » -
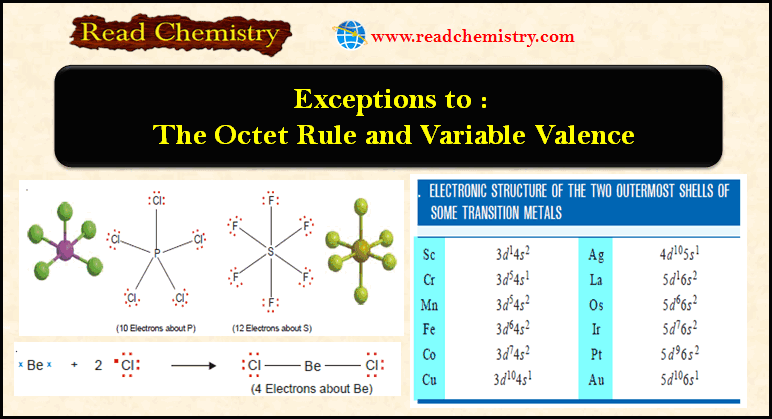
Exceptions to the Octet Rule and Variable Valence
Exceptions to the octet rule – For a time it was believed that all compounds obeyed the Octet rule or…
Read More » -
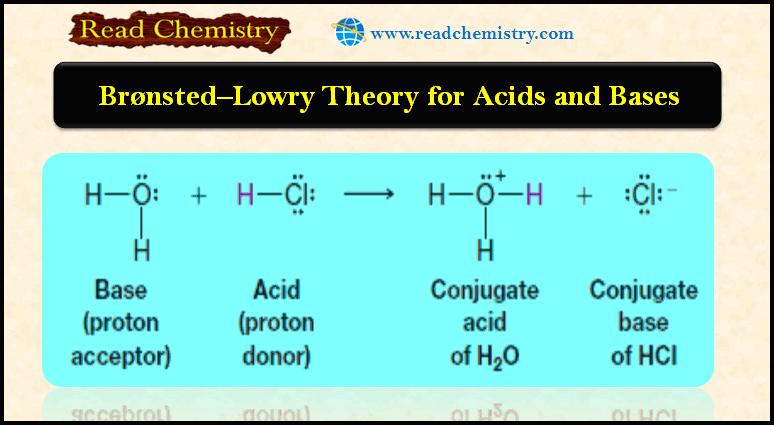
Bronsted-Lowry theory for acids and bases
– In this subject, we will discuss Bronsted-Lowry theory for acids and bases Acid-base reactions – We begin our study…
Read More » -
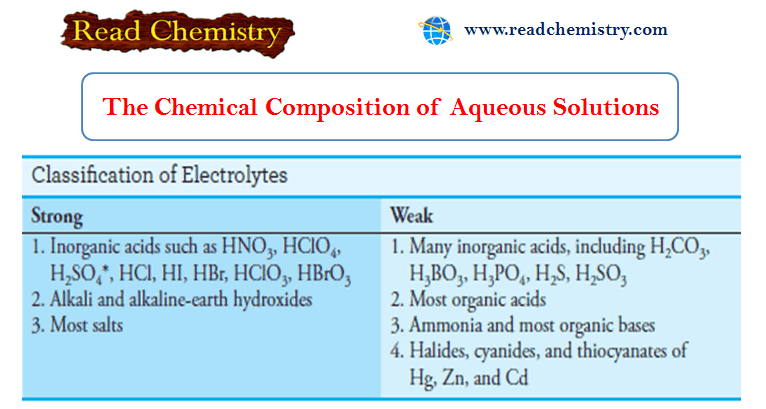
The Chemical Composition of Aqueous Solution
– In this subject, we will discuss the Chemical Composition of Aqueous Solution – Water is the most plentiful solvent…
Read More » -
Concentration of Solutions: Definitions, Formulas, Solved Problems
– In this subject, we will discuss the Concentration of Solutions Concentration of Solutions (Definitions, Formulas, Solved Problems). Concentration of…
Read More »